by, Dr. Sithamalli K. Balasubramanian - Ph.D.

Fig. 3 - Model of anthracene terminal ring. The arrows correspond
to bulges in fig.(2).
Anthracene is unique in this respect as no other published diagram shows these features. The possibility of fixation of the q-surfaces raises interesting prospects. The central ring of anthracene does not show these features.
The case of cyclopropane is equally interesting. The published deformation density diagram {ref.6} of cis - 1,2,3-tricyano cyclopropane (4) shows that the bonding area lies outside the triangle formed by centres of the ring carbon atoms. This phenomenon was used by Coulson {ref.7} to postulate a bent bond. Our model (5) shows that it could also lead to the same result demonstrating that the carbon atom is just the tetrahedral one with angular overlap between bonding areas. There is no need for any special formulation to explain the density diagram
Theoretical calculations of deformation densities {ref.8} predict the negative areas on the inside but not the negative ones outside. The difficulty arises from the assumption of spherically uniform distribution of electron density in the “free” atom. Our model would agree with the diagram (4) in all respects.
The Single Bond; The Inverted Bond:
The tetrahedral single bond does not need any comment and we go on to the inverted bond.(1,1,1) propellane (6) is an interesting case as the M..O theory breaks down here. The axis bond of (6) is an inverted bond. ...top
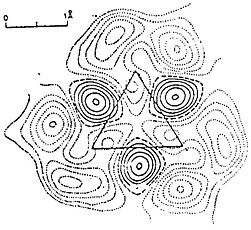
Fig. 4 - Cyclopropane deformation density diagram.
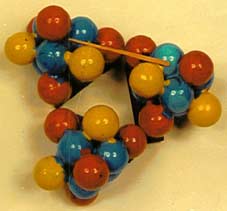
Fig. 5 - Cyclopropane model.
The difference between the normal and the inverted bonds is illustrated in figures (6) and (7). The bond marked ‘a’ is an inverted bond in both while the bond marked ‘b’ is the normal tetrahedral one. On the other hand, (2,2,2) propellane has a normal tetrahedral axis bond.
Theoretical calculations for the inverted bond lead to a zero deformation density in the bonding region expected to lie between the two ‘axis’ carbons in the propellane (6).Wiberg {ref.9} points out that theoretically the propellane (6) should have very little conventional bonding. In other words it should not be capable of existence. He says: “Despite this, the (1,1,1) propellane has a relatively strong bond in the sense that dissociation into singlet diradical involves high dissociation energy”. The compound (6) can in fact be distilled unchanged at about 100ºC.
To compound the confusion the zero deformation density had been found in an experimental system as well {ref.10}. That experimental and theoretical results converge on an absurdity shows that they have the same source of error. In this case the spherically symmetrical distribution of electron density assumed for the ‘free’ atom is the cause of the difficulty, implying that even in a ‘free’ atom the electron densities are directionally oriented along the bonding areas. Such distribution would be an indirect confirmation of our assumptions.
Our model provides an easy solution to the propellane
problem. Essentially the axis bond of the propellane is a q-q bond, the
three peripheral
methylenes being attached to the triangular b-surfaces on the same
face as the q bonds. This was the least stable of all the single
bond possibilities. The observed instability of the propellane (6) which
easily passes into methyl butene on strong heating is because the
axis
bond is essentially weak. Later we show that the q-bond on a rough
approximation can have a maximum of about 69% of the stability of
a tetrahedral single bond. This value should also be the maximum limit
for the strength of the propellane axis bond. Our model is closer
to
the experimental observation than the quantum model.