BASIC PHYSICS AND LAWS RELATING TO PHYSICAL
PROPERTIES OF MATTER
Physics is the Science which covers the various laws which govern the behaviour of matter. These laws are so fundamental that everyone has some knowledge of physics although he may not consider this knowledge as being classified under the title of physics. A knowledge of physics gives us a better understanding of the physical characteristics of the materials we process in the refinery and the energy used in refining. It is not possible to cover the minute details of the physics and cover the extensive subject that are classified under physics. For our purpose,
we will confine ourselves to the basic physics and their application to refining industry.
Definition of Matter
By definition, matter is anything that occupies space and has weight. Matter may exist in different forms like solids, liquids or gases.
Properties of Matter:
Matter exhibits the general properties of impenetrability, inertia and density. In simple terms impenetrability means that no two things can occupy the same space at the same time. A common place example is that some water flows out of a full bath tub when a person enters the tub.
Inertia :
Inertia is another property of matter first} introduced by Sir Isaac Newton. Newtons law states that a body'ln'motion will remain in motion and a body at rest will remain at rest,'unless acted upon by an outside force. Simply, inertia means that a moving body will continue to move unless something is done to stop it or change its direction. Likewise a stationary body will remain stationary unless some force is applied to move it.
Density :
Density is the other property of matter and is defined as weight (mass) of a unit volume. One cubic centimeter of water weighs 1 gram at 0°C and therefore the density of water is 1 gram per cubic centimeter at 0°C. Similarly one cubic centimeter of mercury weighs 13.6 grams at 0°C and therefore the density of mercury is 13.6 grams per cubic centimeter at 0°C.
Specific Gravity :
Many times the density of a liquid or a solid is compared to the density of an equal volume of air. When this is done, the ratio of densities is called specific gravity. Hence specific gravity can be
defined as ratio of the density of the matter and density of water at a certain temperature. Since the rate of expansion of different materials for unit temperature is different, it is necessary to specify the temperature too. In oil industry the-temperature specified is 60°F for English
system (FPS) and 15°C for metric system (CGS). In laboratories temperature used is 0°C. Several types of scales and devices have been developed from time to time to measure the specific gravity of liquids. The most familiar in the refining industry are the API, Baume hydrometers.
The API hydrometer is used for measuring the specific gravity of oil and was developed to give a positive figure even though the density of most of the oil fractions is less than I—the density of water. As the number on the API gravity scale increases, the oil density becomes less. The Baume hydrometer is employed to measure the specific gravity of liquids heavier or lighter than water and is used in the refining industry for caustic soda and acids. The larger the number on Baume scale
the higher is the density of the liquid. The relation between specific gravity and API gravity or Baume is emperical and is related by the following equation.
Degrees API = 141-5 - 131-5
Sp.gr
Degree Be = 145 — 145
Sp.gravity
Basic Properties of Matter :
Nearly all the matter exists in one of the three States - solids, liquids, or gas and may pass from one state to another, depending on temperature and pressure. The most common example is this conversion of ice (solid watery to water by heat and further conversion of water to steam (water vapoury by heat. Another example is the conversion of liquid LPG to gas when pressure is lowered.
The molecules of all matter are constantly in motion and application of heat will increase their molecular motion. Likewise, cooling will decrease the molecular motion. The molecules in solids are packed so closely together that there is barely room for them to move about.
The molecules in liquids are much less compact and therefore they do not take any regular shape, but conform to the shape of vessel containing them. The molecules of gases are even more spaced and have more freedom of movement than liquids. They move about filling the entire volume of the containing vessels. Thearitically, the molecular movements stop at an estimated temperature of -459.4°F i.e. -273°C.
This temperature is called absolute zero or 0° absolute.
The additional freedom of movements of gas molecules gives gases special properties. One of these properties is called elasticity. The gas molecules bounce about colliding with each other and with the wall of the vessels containing them and they bounce back with their original speed and force. This property of bouncing back without loss of energy is called perfect elasticity. Heavier gas molecules move slower than the lighter gas molecules.
Pressure
When gas molecules bounce off the walls of the containing vessel, pressure results. The number of molecules in a container determines the pressure in the container. If more molecules are forced into
a container, then more molecule bombard against the wall of the vessel and higher pressure results. If the temperature is increased, the molecules move faster and more molecules bombard against the vessel walls resulting in higher pressure.
Pressure is most commonly measured in pounds per square inch or kilograms per square centimeter. The air around us is made up of a mixture of gases and at sea level exerts a pressure of 14.7 pounds per square inch or 1.033 kilograms per square centimeter or one atmosphere or 760 mm of mercury. This is termed as absolute pressure which is different from 'gauge* pressure. Normally the pressure gauges are calibrated to read 0 at atmospheric pressure or they indicate the pressure above atmospheric pressure. This pressure is normally referred to as gauge pressure and is expressed as pounds per square inch gauge or kilograms per square centimeter gauge. In normal practice, unless specified as absolute, the pressure readings are assumed to be "gauge" readings. To convert 'gauge* readings to 'absolute' readings, add atmospheric pressure to the gauge readings i. e.
absolute pressure = gauge pressure + atmospheric pressure.
Head is another manner in which pressure is often measured in the refinery, especially in regard to pumps. Head is usually expressed in terms of height of the column of fluid in unit length (meters). For example, if a pump will develop a discharge pressure of 50 meters of water, it will pump a column of water 50 meters high. Knowing that 1 cc of water weighs 1 gram a column of water 50 meters i.e. 5000 cm s high on 1 sq. cm will weigh 5000 grams or will have a pressure of 5000 gms/sq. cm i.e. 5 kg/cm2 . If we take a glass tube 90 cms long, fill it up with mercury, and invert the open end into a dish containing mercury without allowing air to enter the tube, we will find
that the mercury comes down to a level of 76 cms above the level in the dish. The atmospheric pressure acting on the surface of the mercury in the dish is supporting the head of mercury in the tube. In other words, the atmospheric pressure is equal to the 76 cms of mercury head. We know that mercury has a specific gravity of 13'6 / and hence if we were, to do the experiment with water the atmospheric pressure will be able to support a column of water 13'6 X 76 = 1033'6 cms of water.
Vacuum
If from an enclosed container containing air at atmospheric pressure, some air is removed, the remaining air in the container does not exert a pressure on the walls of the container equal to the pressure on the exterior walls. Under this condition, the container is said to be under partial vaccum. If all the air from the container is removed, then the container will be under perfect vacuum. Vacuum is, in other words, negative pressure measured by a gauge. Perfect vacuum is 0 kg/cm2 absolute.
Vacuum readings are generally expressed in millimeters of mercury. Thus 50 mm of mercury vacuum is below atmospheric pressure by an amount equal to 760-50 or 710 mm of mercury. In other words the absolute pressure corresponds to 50 mm of mercury. Since 1 cm of mercury exerts a pressure of 0-0136 kg/cm2 the absolute pressure in the container is 0-0136 X 5 ^ 0-068 kg/cms or 1-03-0-068 = 0-962 kg/cm2 less than atmospheric pressure.
To calculate the pressure exerted by a column of liquid the following formula is used:
hxd = p
where h = height of liquid in cms
d = specific gravity
p = pressure in grams per sq.cm.
Temperature & its Measurements
Heat increases the molecular activity of matter. Temperature is a measurement of molecular activity. Hence temperature is used as a measure for the level of heat. The three most widely used temperature scales are Absolute, Fahrenheit and Centigrade. For normal operations, we will be using centigrade scale.
It is observed that all matters expand on application of heat and the expansion is proportional to the increase in temperature. This property is used for making mercury thermometers. For setting standards, the boiling point and freezing point of water is used. Fahrenheit scale uses 32°F as freezing point and 212°F as boiling point of water giving a 180° span whereas Centigrade scale uses 0° as freezing point and 100° as boiling point giving a span of 100°. The relationship for
conversion of centigrade to fahrenheit is as follows:
°C - (°F - 32) X 5/9
or °F = 9/5 X °C + 32.
Absolute temperature is defined as the temperature at which all the molecular motion ceases. The zero point on the scale has not been achieved, but estimated to be —273°C or —459-7°F. Any centigrade reading can be converted to absolute temp by adding 273° to the reading and fahrenheit reading by adding 4597 to the reading.
In refinery operations the temperature is measured using a thermocouple. The principle used is that the E.M.F. generated by a bimetal is proportional to the temperature. This EMF is compared
with another thermocouple at a constant temperature and is used to indicate the temperature. This is particularly useful in measuring temperatures at higher ranges.
Properties of Gases
As previously mentioned, the molecules in gases are spaced far apart compared to liquids and are very active. They rapidly bombard in the walls of the container in which they are held. This results
in pressure. Because the molecules are spaced apart, gases exhibit many interesting characteristics, the understanding of which will aid greatly in visualising the problems involved in handling them in oil industry.
As explained earlier, gases possess perfect elasticity and the molecules rebound from the walls of the container with the original speed and energy. Each molecule of gas contains at least two atoms with their electrons on the outside of the molecules. The molecules in motion also collides with other molecules and change direction with perfect elasticity.
Boyfes Law
Gases can be compressed and the changes in the volume that results can be accurately predicted by a law called Boyles law. The law states that at "constant temperature, the volume of a gas varies inversely with its absolute pressure". In simpler terms, if the absolute pressure on a gas is doubled keeping the temperature constant, the volume of the gas is reduced to half. In an equation this is represented as follows,
P1 V1
— = — at constant temperature
P2 V2
where P1 is original pressure in absolute unit, V1 the original volume p2 is pressure to which it is subjected to and V2 the volume it will attain under pressure P2
Another law, known as Charles law. relates the effect of temperature on pressure and states that at constant volume, the absolute pressure of gas varies directly with its absolute temperature. For example, in an enclosed container of one cubic meter volume, if a gas at 1 kg/cm absolute pressure and 273° absolute temperature is heated to 546° absolute temperature i. e. twice the original temperature, the pressure will increase to twice the original pressure or 2 kg/cm2 absolute. Mathematically this equation can be represented as follows:
P1 T1
= at constant volume
P2 T2
where p1 = original pressure in absolute unit
p2 = changed pressure in absolute unit
t1 = Original temperature in absolute scale
and t2 = the temperature in absolute scale to which the gas is heated.
A third law, called Gay Lussac's law, relates the effect of temperature on volume and states that at constant pressure the volume of a gas varies directly with the absolute temperature. For example, one cubic meter gas at 273°A will double its volume if heated to 546°A.
Mathematically the law is stated as
V1 T1
— = — , pressure remaining constant
V2 T2
where V1 = Initial volume
V2 = Volume attained
T1 = original temperature in °A
T2 = temperature in °A to which it is subjected to
All the three above laws can be combined into one mathematical equation which takes into account variation of all the three variables. The equation is :
PV
— = a constant K
T
P1V1 = P2V2
T1 T2
where P, P1 and P2 are the pressures in absolute unit
V, V1 and V2 are the volumes
T, T1 and T2 are the temperatures in absolute
Example
To calculate the resultant volume of gas originally 24 cubic meters in volume and under 2 kg/cm2 absolute and a temperature of 273°A when subjected to 3 kg/cm2 and a temperature of 819°A, the equation is used as follows :
P1V1 = P2V2
T1 T2
p1 = 2 kg/cmz absolute P2= 3 kg/cm2 absolute
T1=273°A T2—819°A
V1 = 24 cubic meters ,
2*24 = 3*V2
273 819
or V2 = 2*24*819 = 48 cubic meters
273*3
Partial Pressure
The preceding laws deal with individual gases, but the refinery gases are mostly mixture of gases. A law, called Dalton's law of partial pressures, states that two or more gases held in a single container will exert a pressure equal to the sum of the pressures each gas will exert if separately held in the container.
Still another law called as Avagadros principle states that equal volume of gases under the same conditions of temperature and pressure contains the same number of molecules.
The Dalton's law of partial pressures and Avagadros principle are involved in the important process of stripping lighter hydrocarbons from the heavier hydrocarbons and although stated simply are difficult to understand. The practical application of Avagadros principle will be dealt with in more detail later.
Applying Avagadros principle one cc of hydrogen and I cc of oxygen have the same number of molecules and since the atomic weight of hydrogen is I and that of oxygen is 16, one cc of oxygen weighs 16 times the weight of one cc of hydrogen under the same temperature and pressure. Or conversely, 2 grams of hydrogen and 32 grams of oxygen will occupy the same volume. Experiments have proved that this volume is 22.4 litres at 0°C and 760 mm of mercury pressure (atmospheric pressure). This volume is known as gram molecular volume or commonly referred to as constant volume.
Density & Specific Gravity Of Gases :—
Density is already defined as weight per unit volume. Since gases have very little weight, expressing density per cubic centimeter will be fraction of one and hence the density of gases are expressed in grams per litre (1000 c. c.). Air has a density of 1.293 gms per litre at atmospheric pressure and temperature of 0°C. The relative density of a gas is the ratio of its density to that of air. The easiest way to determine the relative density of any gas is to use the gram molecular weight.
Air consists of 4 parts nitrogen and I part oxygen and therefore its gram molecular weight is
1 X 32 (mol. wt of O2) + 4 X 28 (mol wt of N2 ) = 28.8
5
The molecular formula of propane being Ca Hs , the gram molecular weight of propane is
3 x 12 (carbon) +8x1 (Hydrogen) = 44
gram mol weight of Propane
Relative density of propane = gram molecular weight of air
44
= 28.8 = 1.64
Properties of liquids
Liquids like gases have special properties of their own. The main difference in the three states of matter is in the magnitude of repulsive and attractive forces of the molecules. Gas molecules have
weak attractive and repulsive forces, solid molecules have strong attractive and very little repulsive forces and liquids molecules have considerable attractive forces and yet enough repulsive forces to give them mobility.
Liquid molecules have are much closer together than gas molecules, but there is enough space between them so that they can slide over or around each other. This is what makes the liquid to be fluid, pour easily and take the shape of vessels that contain them.
The attraction that liquid molecules have for each other is what causes them to form drops. Otherwise a single drop of water would spread over the entire surface of anything it is placed on.
This property of liquid is called cohesion. Some liquids exhibit more cohesion than the others. The common example of the property of cohesion is that water sprayed from a hose forms drops when falls down. Also water placed on any surface tends to cling together and forms drops.
Below the surface of a liquid its molecules are surrounded by other molecules. The attractive force of molecules under the surface is applied equally in all directions, whereas on the surface it is applied only downwards and sidewise. Consequently the sidewise pull is greater than the average pull in the molecules below the surface. This gives the surface of a liquid an elastic quality known as surface tension, a form of energy.
Viscosity
A property of liquid widely used in refinery is viscosity. This may be thought as internal friction of the molecules resisting movement relative to each other when subjected to external force. Viscosity is related to cohesion of the liquid molecules for one another and therefore density does not affect the viscosity. The viscosity of a liquid changes with temperature and most of the liquids reduce in viscosity with increase in temperature. This property is used to keep waxy oils and
asphalts in less viscous state and enable the products to be pumped easily. There are different units of measuring viscosity and they can be converted to equivalent readings in another. The viscosities of liquids affect us in pumping of liquids, atomisation of furnace fuels and lubrication of machines.
VISCOSITY INDEX
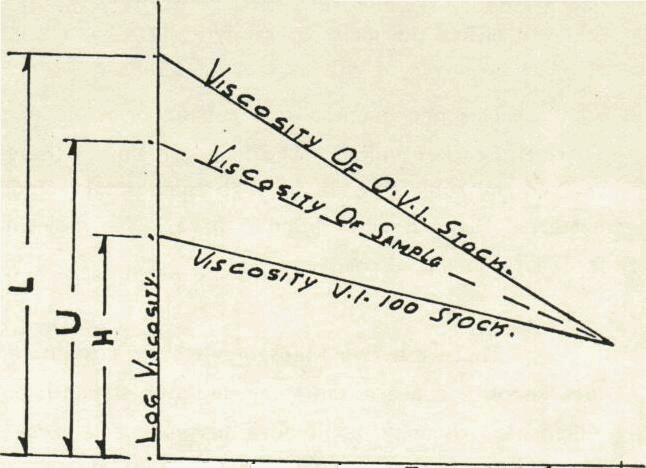
The effect of temperature on viscosity is different for different oils. This property is important for lubrication oils as they should remain sufficiently fluid at low temperature and yet remain sufficiently viscous at higher operating temperature. The most generally used temperature viscosity relationship is developed by Dean & Davis. The viscosity index is based on comparison of viscosity of an oil with that of two reference oil, both of which have the same viscosity at 210° F as the oil under test. The reference oils are prepared from a Pensylvania crude and a Gulf Coast crude and are assumed to have a viscosity index of 100 and 0 respectively.

This Viscosity index is then defined as:
V. I = L — V X 100
L — H
where V = Viscosity of unknown oil at 100° F
L = Viscosity of '0' V.I oil at 100°F
H = Viscosity of 100 V-1 oil at 100°F
In actual practice, tables giving the value of L and H are used for finding the viscosity index of a product. In case of petroleum products, the viscosity of hydrocarbons in the same family like Paraffins, Aromatics, etc., Increases with increasing molecular weight. Therefore, a viscosity reduction is achieved by breaking high molecular weight hydrocarbons into lower molecular weight
hydrocarbons by the process of cracking. The cracking can be achieved either thermally or catalytically.
This phenomenon is the basic principle of the Visbreaker and Thermal Cracker units. The feed stream to these units is thermally cracked by subjecting it to high temperature and pressure in a fired heater. The products obtained have lower molecular weight and there-
fore have lower viscosity.
There are two kinds of viscosity, absolute and kinematic. Absolute viscosity concerns only the cohesive strength of the liquid whereas Kinematic viscosity combines this property with the fluid density. Kinematic viscosity is a better measure of the fluids characteristics in motion. Kinematic viscosity is equal to the absolute viscosity divided by its density. The kinematic viscosity of a liquid is measured by using a viscosimeter. This apparatus measures the time taken for a fixed
volume of liquid to flow through an orifice of standard diameter. The test is determined at constant temperature. The viscosity thus determined is expressed in seconds and the units are saybolt universal seconds (S. S. U.) and saybolt fural seconds (S. S. F.) in the English system and centistokes and stokes in the metric system.
Boiling Point
Liquids can become gases by evaporation. The molecules of liquids on the surface has enough energy to escape and pass into gaseous state. This is true even of cold liquids but the extent of vapourisation will be different for different liquids. When no external source of heat is applied, each molecule has to absorb its heat of vapourisation from the liquid and surrounding atmosphere. This produces cooling effect on the liquid or surrounding atmosphere. An example of this is the cooling effect produced on the body by the evaporation after perspiration.
The rate of evaporation depends on the surface area of the liquid exposed since larger the surface area, the larger is the possibility for the molecules to escape. The cooling increases with increased evaporation. This property is used in refinery to minimise the evaporation loss of volatile products like gasoline and naphtha by storing them in floating roof tanks.
If we pour some carbon tetrachloride in an open vessel the carbon tetrachloride starts vapourising, till all the liquid gets finally converted into gaseous state. In this case, the escaping molecules are
being removed away from the surface and more and more molecules escape from the surface of the liquid. Water kept in an open vessel is also emitting vapours at atmospheric temperature and pressure. If we apply heat to the vessel, more molecules escape and more vapourisation results. The continued heating, till the temperature reaches 100°C, will result in the boiling of water. This temperature is called boiling point of water at atmospheric pressure. Let us assume that the vessel is enclosed. The molecules escaping from the surface fills available space and builds up the pressure in the vessel. On application of pressure it will be observed that the temperature at which boiling starts will be different from 100°C and the boiling point will vary according to the pressure in the container. In other words, boiling point of a liquid varies with pressure. The standard boiling points are expressed in unit of temperature at atmospheric pressure.
Vapour Pressure
When a liquid is confined to a vessel, the molecules still escape from the surface, but they collide with ths walls of the vessel and produce pressure. Some of the escaping molecules will collide with other molecules and may be knocked back into the liquid. When enough molecules escape, the space inside gets saturated as that many molecules are returned back to the liquid as the ones escaping either by collision with the walls of the container or with other molecules and when this
point is reached, the container will be under a certain pressure. This pressure is called vapour pressure of the liquid. Heat increases the vapour pressure of a liquid as more the heat is applied more number of molecules collide against the vessel walls. Therefore, it is necessary to specify the temperature when expressing vapour pressure. The vapour pressure of any liquid at a standard temperature of 100°F is called Reid vapour pressure. The boiling of a liquid starts when the vapour pressure of the liquid equals the pressure exerted on the liquid surface and hence boiling point can be defined as the temperature at which vapour pressure of the liquid is equal to the pressure to which it is subjected to.
Condensation
The reverse of vapourisation i. e. gases becoming into liquid state is called condensation. This can be brought about by compression, cooling or a combination of both. The gaseous molecules contain excess heat called the heat of condensation that must be absorbed in some manner when they become liquids. In the case of compression the resultant liquid absorbs the heat. The common example of condensation by cooling, the condensation of water on the lid covering a vessel containing water that is boiling. The heat from the vapour is absorbed by the lid and condensation results.
Changing of gases into liquids and vice versa is the basic principle underlying the separation of hydrocarbon products in the refinery. Changing of gases to liquid is an important part of refinery
operation as it permits use of smaller pipes and vessels.
Condensation reduces the volume considerably.
Properties of Solids
Hydrocarbon molecules also exists as solids at ambient temperatures - example waxes and asphalts. By application of heat, thereby increasing the molecular activity, solids can be converted to liquids and further to vapours (gaseous state. Solids like Iron are difficult to put in liquid or vapour state. But ice, which is solid water, is easily converted into liquid state, i.e. water, and steam, i.e. gaseous state, by application of heat and is a common example that we come across in daily life.
In refinery the asphalts and sulphur are kept in liquid state by maintaining them at higher temperatures.