Photosynthesis
In this unit of Biology 101 we are going to look at the energy
transformations that take place to manufacture the fuel molecules for living
organisms, and then at the metabolic processes that cells have to burn fuel to
make the ATP needed for all cell work.
The vast majority of living
organisms obtain their fuel molecules, directly or indirectly (recycled, so to
speak) from the process of photosynthesis. The process of photosynthesis
transforms light energy into chemical energy, and uses that energy to produce
the carbohydrate, glucose, from water and carbon dioxide molecules.
The
majority of autotrophs manufacture their organic molecules by the process of
photosynthesis. (Organisms that obtain their organic fuel molecules
pre-formed from the environment are heterotrophs. Animals, fungi, many
protists and many bacteria are heterotrophs.) Most photosynthetic organisms are
plants or protists that contain chlorophyll. Some prokaryotes are also
photosynthetic. The Cyanobacteria have chlorophyll pigments; some Bacteria, such
as the purple sulfur bacteria, have different light-capturing pigments, and
slightly different photosynthetic mechanisms. They are studied in
microbiology.
Note: Not all autotrophs are photosynthetic; a tiny
proportion of our chemical fuel is manufactured by chemosynthetic
organisms. Chemosynthetic autotrophs use energy from chemical reactions
involving inorganic atoms and molecules, such as S, Fe, H and N, to make organic
compounds. Chemosynthesis is restricted to a very few groups of bacteria, mostly
the Archaea. However, chemosynthesis sustains some deep sea-bed ecosystems that
surround sulfur vents.
Once organic fuel molecules are manufactured,
living organisms must degrade the organic fuel molecules to provide energy to
keep their cells alive. These pathways (called cellular respiration) will be
discussed later.
The Process of Photosynthesis
As stated,
photosynthesis involves the transformation of light energy to chemical energy.
The chemical energy is then used to manufacture carbohydrate molecules,
primarily glucose. In eukaryotic organisms, and in the Cyanobacteria, the
process of photosynthesis also produces oxygen. The photosynthetic bacteria
produce organic carbon molecules, but do not produce oxygen. We will discuss the
primary photosynthetic processes of plants only.
Photosynthetic
Requirements
Photosynthesis occurs in all parts of plants that contain
the green pigment, chlorophyll, which is located in the chloroplasts. In
most plants, however, photosynthesis occurs mostly in leaves, where chloroplasts
are concentrated. In the laboratory, we shall look closely at the leaf structure
as it relates to its function on photosynthesis. Let's now turn to this most
important process.
Photosynthesis involves two stages, occurring
in separate locations within chloroplasts. In the light-dependent
reactions of photosynthesis, light energy is transformed into chemical
energy in the form of energy transfer molecules in a series of
oxidation-reduction reactions that transfer electrons and hydrogen from water to
the energy transfer molecule NADP+. The light-dependent reactions are
known as photophosphorylations, because they involve producing
ATP. They take place on the thylakoid membranes of the
grana.
In the light-independent reactions or Calvin-Benson cycle
(or more simply, the Calvin cycle) the energy from the light reactions is
used to manufacture carbohydrate molecules, which form glucose. These reactions
occur in the stroma of the chloroplast.
The overall chemical
equation for photosynthesis is:
Chlorophyll
6CO2 + 12H20 + 686 kcal
---------> C6H12OO6 + 6H20+
6O2
Chlorophyll
(Carbon dioxide + water + light energy ------> glucose +
water + oxygen)
In order to do photosynthesis, water,
CO2, chlorophyll and light energy must be available. We shall briefly
look at each of these requirements before we discuss just how this process of
photosynthesis works.
- Water
Water is the hydrogen and electron donor for the
process of photosynthesis.
Water is obtained from the environment,
absorbed by roots and conducted throughout the plant in the xylem of
the vascular system. Water needed for photosynthesis is but one of the demands
for water in plants.
Light energy is used to split water molecules,
forming 2H+, 2e-, and Oxygen during
the process of photosynthesis.
Carbon Dioxide
Carbon dioxide provides the carbon source for
manufacturing the carbohydrates in photosynthesis.
Carbon dioxide
diffuses from the atmosphere through pores in leaf surfaces, called
stomata, which are formed by a pair of guard cells. The carbon dioxide
then diffuses to the photosynthetic cells of the leaf mesophyll. The
rate of diffusion of carbon dioxide and availability of carbon dioxide often
limit the rate and amount of photosynthesis that occurs in a plant.
Light Waves
Light is a form of electromagnetic radiation. Visible
light is a combination of many wavelengths that we can see as different colors
(of the rainbow) in the range of 380 - 750 nm. Each wavelength is associated
with a specific photon, or particle of energy. In general, shorter wavelengths
have more energy.
The light absorbing photosynthetic pigments do not
absorb all wavelengths of light equally. Some light energy cannot be absorbed
(and is reflected instead) and some is transmitted, or passed through the
chloroplasts. The light waves most absorbed and most useful to photosynthesis
are reds and blues. Not surprisingly, green light is absorbed
poorly.
In the laboratory we shall do an experiment to demonstrate the
absorption of different wavelengths of light by the photosynthetic pigments,
an absorption spectrum.
The Light Absorbing Pigments of Photosynthesis
Chlorophyll is the
primary pigment that absorbs light energy in photosynthesis. In plants, there
are two forms of chlorophyll (a, which has a methyl group, and b, which has an
aldehyde group) as well as important accessory pigments, the carotenes.
Each pigment absorbs certain wavelengths, and collects and concentrates light
energy for the photosynthetic process. The red and blue phycocyanin pigments
can also absorb light and serve as accessory pigments in
photosynthesis.
The Photosystems
The photosynthetic pigment
molecules do not work alone. They are arranged on the thylakoids of the
chloroplast in clusters of about 300 pigment molecules (plus some other
molecules) in a light-harvesting complex (sometimes called the antenna
complex) that gathers and transfers energy to a reaction center that
has a special chlorophyll a molecule. The reaction centers of
Photosystems I and II are activated by slightly different wavelengths of
light.
There are two such light-harvesting complexes found in the
chloroplasts, called Photosystem I and Photosystem II. In
addition to the light-harvesting complexes, each photosystem has a primary
electron acceptor, which accepts the electrons released from
chlorophyll a, and an electron transport system as well as
the.
Chloroplasts
The pigments needed for photosynthesis are located in
the chloroplasts. Recall that the chloroplast has a double membrane with a
series of internal stacked membranes. Light energy is captured by the pigments
located on the special membranes in the chloroplast called thylakoids,
which are folded into disk-shaped stacks called grana. The interior
compartments of thylakoids serve as reservoirs for hydrogen ions
(H+) that are needed to produce ATP.
The
reactions of photosynthesis that are involved in the transformation of light
energy to chemical energy, the light-dependent reactions, occur on the
thylakoid membranes of the chloroplast. Each thylakoid has thousands of the
two photosystem complexes.
The reactions needed to produce
carbohydrates occur in the stroma region of the chloroplast. Enzymes
are located here. These reactions are known as the Calvin-Benson cycle
or light-independent reactions.
Electron Transport System Molecules (Energy Transfer Molecules)
As
discussed previously, many chemical reactions of metabolism are
oxidation-reductions that utilize a chain of electron transport molecules.
Electron carriers make it possible for us to trap and use solar
energy in photosynthesis. In the process of photosynthesis, the electron
transport carriers are embedded in the thylakoid membranes.
The
most important energy transfer molecule in photosynthesis is
NADP+.
The photosynthetic pathways.
The
two "stages" of photosynthesis are linked by the products of the first stage,
the light-dependent reactions. These products are ATP and NADPH.
Stage
I: Light-Dependent Reactions.
- The light-dependent reactions transform light energy into chemical energy
that is trapped and carried by ATP and NADPH to the Calvin Cycle.
- The light-dependent reactions require chlorophyll and occur in the
thylakoid membranes of the grana of the chloroplast.
- Light energy is also used to split water (Photolysis of water)
into:
H2O -----> 2H+ + 2e- +
1/2O2,
This produces oxygen and
provides electrons and Hydrogen for the reduction of NADP to NADPH (NADP gains
H+ and electrons; the water is oxidized because it loses the
H+ and e-)
- The light-dependent reactions are photophosphorylations because
they involve using light energy to make ATP
- The light reactions consume H2O and produce ATP, NADPH and
O2 as byproduct.
Stage II: Calvin Cycle
- These photosynthetic reactions do not use light energy for their energy
source. They use the ATP produced in the light reactions for their energy
source, and the energy transfer molecule, NADPH to provide Hydrogen and
electrons for a high-energy reduction.
- Carbohydrate molecules are produced in Calvin Cycle in the stroma
of the chloroplast.
- In the Calvin cycle, carbon dioxide combines with a 5-carbon sugar
(Ribulose bisphosphate) and undergoes a reduction to form 3-carbon molecules.
These 3-carbon intermediates can be used to regenerate the 5-carbon sugar or
metabolized to form the carbohydrate, glucose.
The
Specific Mechanisms of Photosynthesis
The Light Reactions - Cyclic
and Non-Cyclic Photophosphorylation)
1. Non-Cyclic
Photophosphorylation
Uses
- Photosystem I
- Photosystem II
- Electron Transport System
Inputs
Water
Light energy
Energy Transfer Molecules
ADP and P
NADP+
Outputs
ATP
NADPH (reduced form) (from NADP+, the oxidized form)
O2
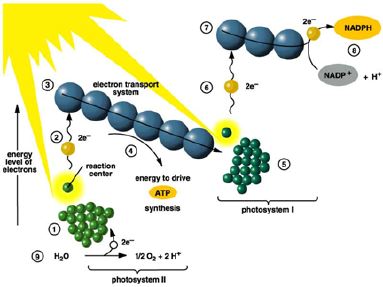
Non-Cyclic
Light-Dependent Reactions of Photosynthesis
How it works:
- Light energy hitting photosystem II excites electrons in the p680 reaction
center causing the chlorophyll molecule to lose electrons. They are picked up
by the electron carriers and passed “down” slowly releasing energy as they go.
Some of this energy is used to produce a H+ gradient within the
thylakoid. This gradient ultimately drives the mechanism that produces ATP by
chemiosmosis (see a bit later).
- Light energy is also hitting photosystem I and exciting its reaction
center chlorophyll electrons. They are picked up by other electron carriers
and transferred to NADP+, which will also pick up a H+
forming NADPH.
- The electrons released from photosystem II, once they have passed through
the electron transport system, are used to replace the electrons lost by
photosystem I that were used to form NADPH.
- The electrons lost by photosystem II are replaced by the splitting of
water. Water’s electrons are passed to photosystem II; its H+
is used in the H+ gradient (and can be passed to NADP+)
and its oxygen is released as oxygen gas molecules.
To summarize,
the low energy electrons from water are elevated in energy by passing through
both photosystem II and photosystem I and trapped by NADP+ where
their potential energy will be used for the high energy reduction of carbon in
the Calvin cycle. Along the way, ATP, needed for the endergonic Calvin cycle,
is also produced.
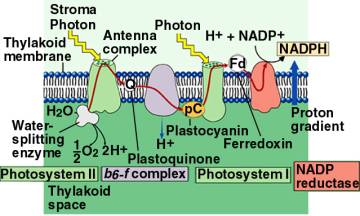
Orientation of Electron
Transport Molecules and Photosystems in the Thylakoid Membranes
2. Cyclic Photophosphorylation
Uses
Photosystem I
Electron Transport System
Inputs
Light energy
Outputs
ATP (from ADP and P)
Cyclic Photophosphorylation only uses
Photosystem I and produces ATP. In this process, electrons released from the
chlorophyll p700 are returned back to Photosystem I after passing through the
chain of electron carriers.
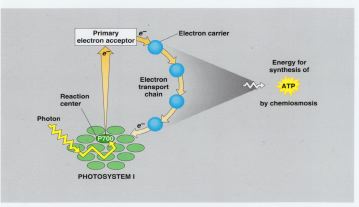
Cyclic
Photophosphorylation
Before we go much further, just how is the
ATP, produced in the light-dependent reactions really made?
The
Chemiosmotic Theory of ATP Synthesis
We have discussed that electrons
released from molecules (such as chlorophyll) can travel down an electron
transport system, releasing their energy in controlled bits. This energy, as we
have said, can be used for the synthesis of ATP.
In photosynthesis, the
molecules of electron transport system are located in the thylakoid membrane.
The energy released from the electron transport system is used to move Hydrogen
ions (H+) from the stroma into the inner thylakoid compartments by
active transport. This concentration of H+ in the inner compartment
of the thylakoid establishes a concentration and a charge gradient in the
thylakoid compartment that has a high potential energy.
The accumulated
H+ ions diffuse through ATP synthase protein complex channels in the
thylakoid membranes that are coupled to ATP synthesis. As the H+ ions
flow down the gradient in the protein channels, their energy is used to make ATP
from ADP and P on the other side of the thylakoid membrane in the
stroma.
Note: Peter Mitchell won a Nobel prize in 1978 for determining
the chemiosmotic theory of ATP synthesis. ATP is synthesized in the mitochondria
during cell respiration by a similar mechanism.
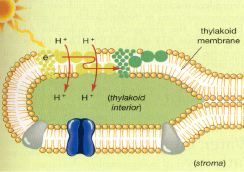

Chemiosmotic synthesis of ATP in
the Thylakoids
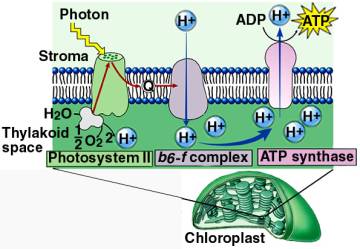
Chemiosmosis in Photosynthesis
- a second view
The Calvin-Benson Cycle and Carbon Fixation
The second set of
reactions for photosynthesis is known as the Calvin cycle, or the
light-independent reactions. They occur in the stroma region of the chloroplast
and use the products formed during the light reactions of
photosynthesis.
There are many parts to the Calvin cycle:
- Carbon Fixation
- Reduction
- Regeneration
- Surplus (Output)
The requirements for the Calvin cycle
are:
- Carbon dioxide (CO2)
- NADPH from the light-dependent reactions (reducing power, source of
electrons and the hydrogen source)
- ATP from the light-dependent reactions (energy source)
- Ribulose bisphosphate, regenerated in the cycle
- Appropriate enzymes for each step in the cycle. Of these, Ribulose
bisphosphate carboxylase (Rubisco) is especially
important.
The Metabolic Intermediate in Process:
- G3P (Glyceraldehyde 3 Phosphate)
The Calvin cycle
produces:
- Glucose (carbohydrate)
- Ribulose bisphosphate, regenerated in the cycle
By
the way, the process we are discussing was determined using radioisotopes of
14C. The researchers (Calvin, Benson and others) won a Nobel prize
for this. This pathway is called 3-carbon photosynthesis because of the
3-C G3P intermediate to distinguish it from an alternative pathway known as
4-carbon photosynthesis, to be discussed in a bit. To some extent,
the Calvin cycle looks like a carbon cut-and-paste dance. Well, it is. It's easy
to follow the maze if one counts carbons, even in more detail than presented in
your text. It also helps to remember that without this happening, you'd
starve!
Note: Only the carbon backbone of the molecules are shown in the
diagrams, except where other atoms are critical to the structure!
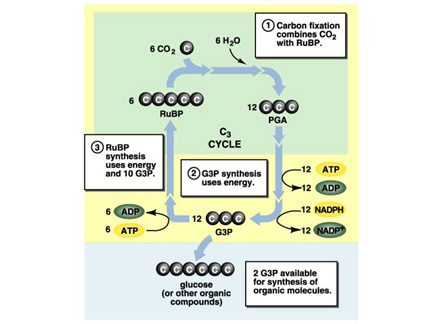
Carbon Fixation and the Reduction Phase of the Calvin
Cycle
This must repeat 6 times to form 1 glucose
CO2 + RuBP (Ribulose bisphosphate) ----> Hexose Phosphate ---->
C + C ----> C ---->
C C
C C
C C
C-P C
C-P
2 PGA (Phosphoglyceric Acid) ----> 2 G3P* (Glyceraldehyde 3 Phosphate)
(+ 2 ATP + 2 NADPH) ----> (+ 2 P + 2 ADP + 2 NADP+)
2 of C ----> 2 of C
C C
C-P C-P
A
balanced Reduction uses: 6CO2 + 6 RuBP + 12 ATP + 12 NADPH to produce 12
G3P.
* Although not diagrammed, the G3P has 1 more H atom in its
structure than PGA. The H was donated by NADPH during the reduction step. This
forms an aldehyde molecule, rather than an organic acid. Molecular diagrams are
available on request.
Regeneration of Ribulose bisphosphate (RuBp) phase
This
will use 10 of the 12 G3P molecules from the reduction phase.
10 G3P + 6
ATP ----> 6 RuBP + 6 H2O (+ 6 ADP)
C C
C C
C C + ATP ----> RuBP
C
C
C
C C
C C remove "head"
C
C
C C C C
C C C C
C C C + ATP ----> RuBP
C C
C remove "head" C
C C
C C
C
C
C
C C + ATP ----> RuBP
C C
C C
C C
C C
C C + ATP ----> RuBP
C
C
C
C C
C C remove "head"
C
C
C C C C
C C C C
C C C + ATP ----> RuBP
C C
C remove "head" C
C C
C C
C
C
C
C C + ATP ----> RuBP
C C
C C
Note: Water, ADP and P are given off in the process.
The Surplus Phase - Producing Glucose
The remaining 2
molecules of G3P are converted into one Glucose molecule
2 G3P ------------------------------------------------> 1 Glucose
1. 2. 3. 4. 5.
C + C ----> C-P ----> C -----> C=O ----> C=O
C C C C=O C C
C-P C-P C C C C
C C C C
C C C C
C-P C-P C-P C
(+ P) (+ P)
1. G3P (Glyceraldehyde 3 Phosphate)
2. Fructose 1,6
bisphosphate
3. Fructose 6 phosphate
4. Glucose 6 phosphate
5.
Glucose
Note The =O is shown only to indicate the isomer rearrangement
that occurs in step 3 to 4 between fructose-6-phosphate and
glucose-6-phosphate.
What comes after
Photosynthesis?
Although glucose is the typical end product of
photosynthesis, and as we shall discuss, the primary fuel molecule for living
organisms, plants are capable of synthesizing all of their organic molecules
from photosynthetic intermediates, notably G3P and glucose
phosphate. Plants are more versatile in their synthetic abilities than are
animals. In addition there are whole groups of plant products, called
secondary metabolites, synthesized directly or perhaps as by-products of
plant activity.
Some of these metabolites are protective in nature, such
as toxins; some, such as lignin, are used in plant structure.
How
Productive is Photosynthesis?
We have seen that it takes 18 ATP and 12
NADPH to make one molecule of glucose. Much of the light energy that hits the
surface of plants is not absorbed and not available. And much of the light that
hits earth does not hit photosynthetic surfaces of plants.
Plants also
need water and carbon dioxide for photosynthesis. The stomata found in the leaf
surface that permit CO2 to diffuse into the leaf also permit the
diffusion of water and O2 out of the leaf. This loss of water
can be significant. As much as 90% of the water absorbed by the plant is lost
this way. (This evaporation of water through the stomata is also used by the
plant to generate a tension that serves to pull water up through the xylem from
the roots to stems and leaves, so it is not all a negative thing for the
plant.)
Photorespiration
Under very hot and dry conditions,
many plants must close their stomata to minimize water loss. During these times
the ratio of oxygen to carbon dioxide in the leaf increases, and this favors a
process called photorespiration.
Rubisco, the enzyme that brings
CO2 and RuBP together, works only when the concentration of
CO2 is high relative to the level of O2. When
CO2 levels drop, the enzyme, Rubisco, combines RuBP with
O2 and the Calvin cycle is disrupted. Photorespiration decreases the
photosynthetic output of the plant.
C-4 Photosynthesis
Some
plants in hot, dry environments have evolved mechanisms to minimize
photorespiration. When CO2 diffuses into a mesophyll cell, it is
combined with a 3-carbon compound, PEP (phosphoenolpyruvate), forming a 4-carbon
acid, Oxaloacetic acid.
This 4-carbon acid is then transferred to the
bundle sheath cells of the leaf. This is a more efficient trap for carbon
dioxide since the 4-carbon acid can accumulate during non-light periods,
concentrating carbon dioxide when photosynthesis cannot occur, and can be used
during periods of low moisture when stomata are closed to prevent water loss.
Such plants have a photosynthetic mechanism called C-4 photosynthesis
(because of the 4-carbon acids formed).
C-4 plants also perform the
two stages of photosynthesis in separate cells to keep O2 away from Rubisco. The
light reactions (which produce oxygen) occur in the leaf mesophyll cells that
surround the bundle sheath cells of veins. The Calvin cycle occurs in
specialized bundle sheath cells whose chloroplasts have very few thylakoids.
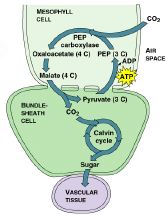
C-4 photosynthesis is highly productive in hot and dry environments. The
world's most productive plants are C-4 plants. However, regenerating PEP
requires ATP, so C-4 photosynthesis is not always more productive than the C-3
pathway. Unfortunately, the pathway is genetic, so plants can't choose.
CAM - Another Conservation Mechanism
Some other plants
minimize water loss by reversing the time of day when stomates are open. Plants
that have reverse stomatal opening can also store CO2 in 4-carbon
acids. They do not, however, have photosynthesis separated into two different
cells. In the daytime, this CO2 is released for "normal" C-3
photosynthesis, while the stomata can remain closed to prevent excessive water
loss. The name, CAM (for Crassulacean Acid Metabolism) is derived from the types
of plants in which it was first discovered, Crassuleans, and for the fact the
CO2 trapped forms acids.
Bacterial
Photosynthesis
Some bacteria have photosynthetic pigments and a process
of photosynthesis. There are some differences, however:
- Bacterial chlorophyll is different from the chlorophyll of other
photosynthetic organisms.
- Bacteria do a just cyclic photophosphorylation. They can not do
photolysis, and do not produce oxygen.
Chemosynthetic
Bacteria
As mentioned in the introduction to this chapter, some bacteria
can use inorganic molecules such as Fe++, NH4+,
S and H to provide energy to "fix" carbon (that is make organic compounds from
inorganic sources). In the scheme of life, chemosynthesis plays a small part in
energy acquisition. Yet the environmental role of chemosynthetic bacteria, such
as in the nitrogen cycle, is critical.










