I.1. Energy and living organisms: The carbon cycle
I.2.The carbon cycle in aquatic (marine) system
Go to ECOMAMA syllabus home page
Living organisms basically take up and transform energy from the environment. The autotrophs (plants) take up inorganic components from the environment: mostly CO2, H2O and nutrients such as P, N, Si necessary for the build up of organic material. Organic, contrary to inorganic material, consists mainly of carbon. Plants contain chlorophyll, a molecule specially constructed to capture the energy provided by sunlight. Using the sunlight as energy source, plants build up their own organic matter (carbohydrates, proteins, fatty acids) through primary production.
Heterotrophs (animals) cannot convert inorganic matter into organic matter, but feed on the organic matter already produced by primary production, either directly by eating plants, or indirectly eating other organisms, either alive and/or dead. They take up organic matter as food (ingestion, I), digest it into simple molecules (e.g. proteins into amino acids), and transform this partly into their own biomass (production, P). Another part of the ingested food is oxidized (respiration, R), to produce energy and CO2. This energy is needed to perform the digestion and transformation of food into their own biomass and to perform other live functions (locomotion, transfer of electric signals in nervous systems etc). A certain fraction of the food cannot be used and is excreted (excretion, E).
I = P + R + E
When a living organism dies, it's biomass is reconverted into inorganic compounds (nutrients) by the heterotrophic activity of bacteria (see below). These nutrients can again be recycled and used by primary producers.
Not only the heterotrophs, but also the autotrophic organisms respire and excrete fractions of their produced organic matter. In fact, the energy captured by the plants from the sunlight is transformed into chemical energy on the one hand, which is “stored” in the body mass, and on the other hand, into other more immediately used energy forms, such as the energy needed for locomotion or for electronic nerve signals.
The reactions below show that photosynthesis and respiration are in fact reverse processes: photosynthesis constructs organic matter out of inorganic matter using sun energy, respiration breaks down organic matter into inorganic matter and frees energy. The first process takes place only in plants, the latter in both plants and animals.
|
+ sun energy |
photosynthesis | |
|
6 CO2 + 6 H2O + nutrients |
<==> | C6H12O6 (organic matter) + 6O2 |
|
+ energy |
respiration |
The ratio between energy stored as biomass (B) and respired (R) decreases with the size of the organism and generally follows the relationship: R= kB2/3 (k is a constant). This means that, the bigger an organism the more energy it stocks under the form of biomass, relative to what it respires.
Another characteristic that varies with the size of organisms is their abundance (= concentration, also called density): in sea water bacteria occur in abundances of 109 - 1012 litre-1, while the abundance of e.g. copepods (much larger) is maximally 1 liter-1.
The fact that bacteria are so abundant and convert such relative high amounts of energy allows them , through their heterotrophic activity, to recycle the organic matter of dead plants and animals into inorganic components. Just as the heterotrophic animals, they take up organic matter, and respire a (relatively large) part of it, while the rest is stocked as biomass.
Within each ecosystem, the co-occurrence of primary production on the one hand and heterotrophic activity on the other hand leads to the cycling of energy and organic matter in the ecosystem. Because carbon is the main element in this cycling, it is generally called the carbon cycle.
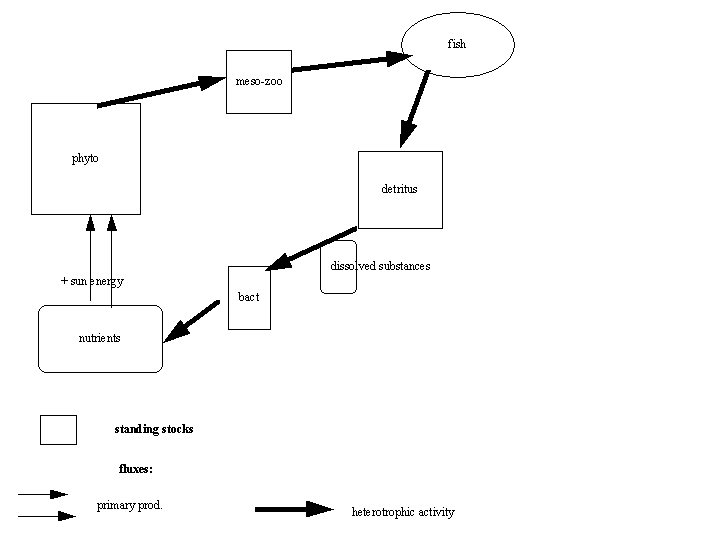
In the water column, the main performers of this spectacular transformation of energy are the planktonic organisms. Planktonic organisms are defined as those organisms which live in the water column, and cannot actively swim against the water currents. Plankton compresses both vegetal algal species, the phytoplankton, and animal species, the zooplankton. Phytoplankton species in marine ecosystems mostly belong to two major groups: the diatoms, which are generally large (5 - 100 µm) cells, having a silica shell, and the flagellates, which are generally smaller than diatoms (2 - 40 µm), and have one or more flagella, but no silica shell. The most frequently studied zooplankton organisms in marine ecosystems are the mesozooplankton (organisms between 100 - 1000µm in size), because of their important biomass encountered in many marine systems. Within the mesozooplankton, copepods usually dominate. To understand the structure and functioning of pelagic systems, it is necessary to quantify the abundance of various planktonic components, as well as their activity. The phytoplankton performs primary production, while the zooplankton feeds on the organic matter produced by the phytoplankton, and in turn serves as food for higher trophic levels: fish.
These in turn serve as prey to top predators for example seabirds. When phytoplankton and zooplankton die, the dead organic matter (detritus) is further decomposed into dissolved components by the action of microplanktonic organisms (see below) yeasts and bacteria, and further recycled to nutrients by the heterotrophic activity of the bacteria.

Fig 1.1 Transformation of energy in an aquatic ecosystem: the C-cycle
The various groups of organisms and components acting in the transformation of energy within a system are called the compartments, and their (bio)mass present in the system is the standing stock (boxes in Fig.1.1).
To obtain a quantitative measure of the energy flow through the various compartments of an ecosystem, we have to quantify these standing stocks and fluxes. Because carbon is the basic element of this energy flow, and to have a uniform measure throughout the different components, this quantification is usually undertaken in carbon. Standing stocks are expressed in carbon weight per unit volume of water (e.g. mgC m-3); fluxes in C- weight per unit volume of water per unit time (e.g. mg C m-3 d-1).
Because both the rate at which chemical reactions take place, and the activity of living organisms are influenced by the environmental conditions in which they occur, we will also need to quantify a number of physico-chemical variables, such as temperature (T) and salinity (S).
The final aim of these quantifications is usually not only to get an idea of how energy runs through a given system during the time of the study, but to be able to deduce mathematical relationships describing the processes between the stocks and fluxes, and the factors influencing them.
For example, as shown in fig.1.2 the ingestion rate (I) of a meso-zooplankton organism can vary with the concentration of phytoplankton (C) following a saturation type curve, which can be mathematically described as a Michaelis- Menten curve:
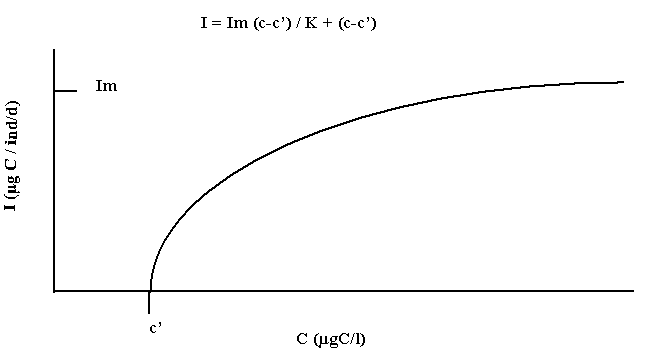
Im: maximum I K: half saturation constant c’: concentration at which I stops.
Fig.1.2 Ingestion rate of a mesozooplankton as a function of phytoplankton concentration
These relationships can then be combined in an ecosystem model, which allows predictions to be made on developments in the ecosystem (or one of its components) as a function of changes in one or more factors. These changes can for instance be the change in salinity caused by rainy and dry seasons, or man- induced changes such as increasing loads of detritus input caused by deforrestation and subsequent increases in erosion. Such models can be used as tools in the management of ecosystems, as they allow the testing of the probabilities of various outcomes obtained from various management scenarios.
The above description of the carbon cycle in marine systems is an oversimplification. Several other components and processes act in the carbon cycling in marine systems, the most important of them being:
in many marine ecosystems, mixotrophic organisms, which can both perform primary production and feed heterotrophically on organic matter are abundantly present. These organisms are mainly planktonic, such as the cynobacteria and dinoflagellates.
In many cases the phytoplankton is not only eaten by (meso) zooplankton, but also by micro (zoo)plankton. The microplankton consists of organisms such as ciliates, tintinnids, but also mixotrophic organisms as with some of the dinoflagellates.
The degree in which mesozooplankton on the one hand and / or microplankton on the other hand develop determines the way in which the ecosystem functions, in other words through which pathways the carbon (and energy) flow is passing through the system. At the basis, this is influenced by the turbulence of the water, and the nutrient concentrations and ratio’s between these nutrients. In general terms, small phytoplankton cells can take up nutrients faster than large phytoplankton cells because they have a large surface / volume ratio. In less turbulent water, where nutrient import from outside the system by turbulence is low, nutrient concentrations are generally low, and large phytoplankton species can not compete with small phytoplankton species in nutrient uptake. This leads to the development of small phytoplankton species, which are inefficiently fed upon by mesozooplankton. Microplankters, on the contrary, do feed efficiently on small phytoplankton and develop large populations. Some mesozooplankton species do feed on microzooplankton, however, and as such, so a (limited) transfer to the higher trophic levels also occurs. The bulk of the primary production is used by micro-organisms, or becomes detritus and is finally recycled by bacteria. This type of biological passageway for carbon cycling is called the microbial loop or web (Fig 1.3b).
In turbulent systems, the import of nutrients by turbulence is higher, and in those conditions, large phytoplankton species (mostly diatoms) can compete with small species for nutrient uptake. So in these systems, large phytoplankton species develop, and can serve directly as food for the mesozooplankton.
The resulting development of mesozooplankton populations also allows the development of higher trophic levels. These are the sorts of systems that may be exploited as fishing grounds. This trophic pathway is called the herbivorous food web (Fig.1.3a).
In reality, a wide range of ‘sorts’ of ecosystem functioning exist, of which the ‘herbivorous food web’ and the ‘microbial loop’ are the two extremes.
The above only considers phytoplankton as the food for the mesozooplankton and micro(zoo)plankton. In many cases however, the zooplankton has a broader choice of food items. Both micro- and mesozooplankters are suspension feeders, which means they feed on particles suspended in the water. Besides phytoplankton cells, also present are dead plant fragments (phytoplankton and benthic algae), remains of dead animals (zooplankton and higher organisms) as well as bacteria. The term seston stands for all particulate matter suspended in water. Particulate means that the particles are > 1um (as opposed to the materials in solution which passes through a 1 µm filter). Suspended means that these particles cannot actively maintain their position against a current.
So the seston is composed of:
- living organisms (plankton): phytoplankton, zooplankton, bacterioplankton
- dead particles: detritus
e.g. carbonates, sand, minerals
Sometimes, particles which were present (settled) on the bottom of the ecosystem are resuspended into the water column. These resuspended sediments are usually a mix of both organic and inorganic components.
In practice, bacteria and zooplankton are not often considered under seston studies, which concentrate mainly on phytoplankton, detritus and inorganic particles (e.g. sand).
Besides counting the seston components, which is a labour intensive method, a number of chemical measurements can also be used to quantify the (bio) mass of seston components.
Analysis
Quantifies
- Dry weight
Total particulate matter (organic + inorganic)
- P.O.C
Particulate organic carbon: a measure of the amount of organic matter (living phytoplankton + detritus).
living phytoplankton
The (relative) abundance of the different types of particles present in the seston depends on the ecosystem, and is strongly influenced by the location (depth) of the system.
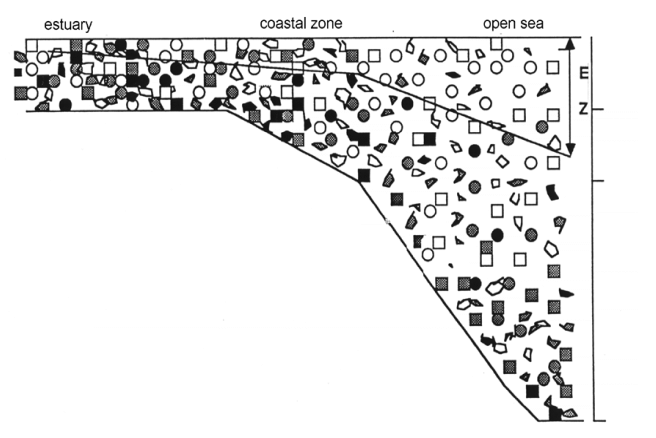
Fig. II.2. Represents the abundance of various types of particles in a transect going from an estuary through the coastal zone towards the deep sea.
In the deep (open) sea systems, the phytoplankton receives enough light for primary production in the upper water layers only. When the cells die, they rapidly sink to the bottom, because turbulence is low. Particles on the bottom (detritus, sediments) are not strongly resuspended, so that the seston in the upper water layers consists mainly of phytoplankton (and zooplankton).
In the coastal zone, the tidal movements and wind action creates a higher turbulence because of the limited depth here, detritus and sediments are resuspended regularly in the water column, and are more abundant than in the open sea. There is also a substantial import of material from terrestrial origin, such as plant detritus, eroded soil etc. The seston in coastal zones is a mixture of phytoplankton, detritus and resuspended sediments.
In estuaries, the action of the tidal currents is very strong because of the limited depth and space through which the water has to pass at each tidal cycle. Here a lot of detritus and sediments are kept in suspension, and total particulate matter concentration is very high. The high import of particulate matter from terrestrial origin by the river inflow contributes extra to the high particle concentration. Because of this high particle concentration, turbidity is high (light penetrates only through a very limited depth into the water), and primary production is light limited. As a consequence, phytoplankton abundance is relatively low, and the majority of the seston in estuaries consist of detritus.
So in most systems, the particulate matter available to the zooplankton as food consist of various types of particles. Depending on the (relative) concentration of these various seston components, the zooplankton can feed on the various particles with varying intensity (cf. Zooplankton course).
Figs. I.3a. & b. give a more complete picture of the carbon cycling in marine ecosystem, indicating the various seston components and interacting components from outside the system. In the case of the herbivorous food web (Fig. III.3 a), the major part of the primary production goes to the development of zooplankton and higher trophic levels. In the case of microbial web (Fig. III. 3b) most of the primary production is used by bacteria and microplankton, while mesozooplankton and higher level development is limited.
Because these practical exercises are focused on the C-cycling in the pelagic zone, the above explains mainly the role of planktonic organisms. It should be realised however, that the benthic components also play a very important role in the C- cycling of aquatic ecosystems. Biogeochemical processes taking place within the bottom and on the bottom surface can moreover provide an important interaction between the benthic and the pelagic comportment e.g. nutrient release from the bottom into the water, consumption of phytoplankton by filter feeding benthic organisms).
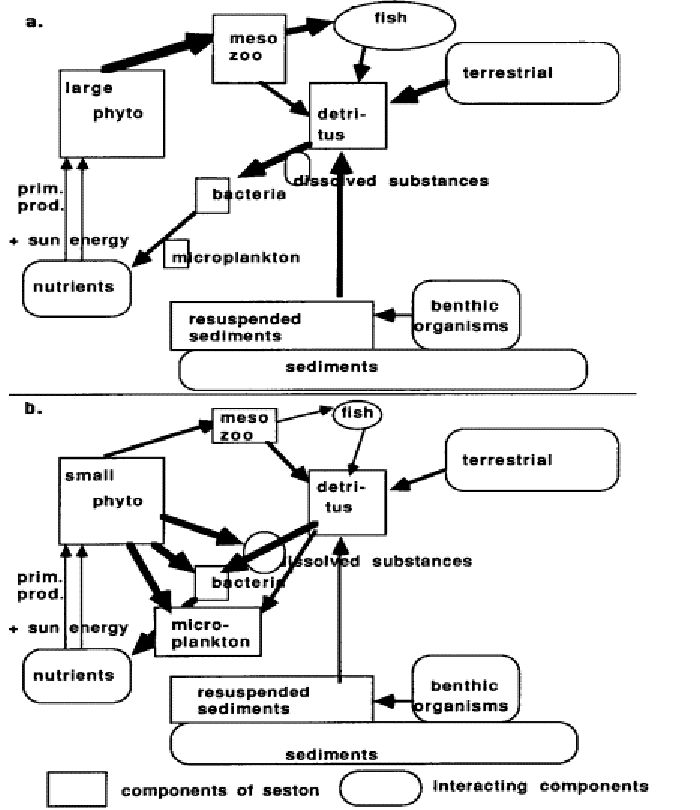
Fig 1.3. Carbon cycling in a marine ecosystem
To make adequate measurements of any stock or process in an ecosystem, one has to rely on samples, so each time research on a specific topic in a ecosystem is started, the question arises : “what is the sample size we need to take?”An important phenomenon in this regard in an aquatic ecosystem is the fact that the abundance of organisms (and particles in general) increases with their size. For example, there are millions of bacteria in 1 ml of seawater, but there are generally only a few copepods per litre of seawater.
Because of this adequate sample size is dependant on the size of the things you want to study. As can be seen from FIG II.2, it is feasible to obtain a representative sample of e.g. abundance of flagellate cells (pictured in grey) in 1 l sample, but this is not sufficient for sampling copepods (in black). This requires at least 50 l of water.
Fig.II.2. Representation of abundance of various particle size in natural samples
The example above illustrates that, the larger the sample size, the better the chance to make a correct estimate of the abundance of a component.
We can test what is the minimum sample size needed for a specific component by taking a number of replicate samples and analyzing them.
Fig II.3 shows an example of tintinnid abundance counted from a sample taken in a marine lagoon, ranging from 1 to 10 litres in size (average of 3 replicates). We see that the average obtained fluctuates strongly for small samples, but stabilizes around 16 ind L-1 from 7 litres onwards.
In this example, we would conclude that 7 litres is the minimum sample size needed to quantify the abundance of tintinnids, in this specific ecosystem, correctly.
We also notice that the standard deviation (see the bars in Fig. II.3) around the mean, becomes smaller as sample size increases. This is another representation of the fact that each replicate sample separately is less likely to deviate strongly from the mean (of the samples), but also of the real value, as sample size increases. A simple way to quantify the standard deviation in relation to the mean is the coefficient of variation: c.v.
c.v. = (s* 100) / x
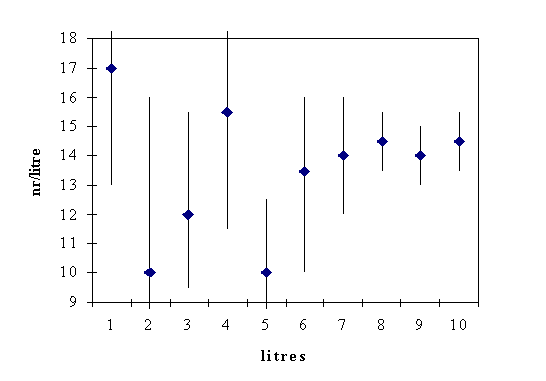
Fig.II.3. Abundance of tintinnids obtained from counts of various sized samples
In principle, one should make a series as shown in Fig.II.3 each time one starts to study a new ecosystem. In practice however, one knows from experience which are ‘safe’ sample sizes to work with in most cases. The equipment used for sampling for specific seston components is adjusted to these sample sizes.
For the smaller components, bacteria and phytoplankton, water is usually collected with a Niskin bottle, which has a volume of 10 - 50 litres. From this volume smaller sub samples are usually taken for different specific analysis, such as bacteria abundance counts (a few ml is sufficient here) or microscopic phytoplankton cell counts (0.5 - 2 L) or analysis of chemical components representing specific fractions (e.g. chlorophyll a; also 0.5 - 2 L).
For mesozooplankton, a volume of 50 - 200L needs to be sampled, and this is done by filtering this volume of water through a 50µm net, and collecting the zooplankton trapped in the net. If we want to quantify the abundance of large zooplankton organisms, such as fish larvae, we need to sample a few thousands litres, and this is done by dragging a large net (with mesh size e.g. 300 µm) behind a ship.
As mentioned in the process of sample analysis, sub samples from the Niskin bottle are often taken for different analysis. This poses the question: how big do the sub samples have to be? In fact, the question of sample size will return several times in the process of the different steps of sample analysis. At each step, the same principles explained above for the field sample, hold. The specific applications will be outlined with each specific analysis below.
Depending on the specific analysis to follow, the samples will be treated directly after sampling in various ways: for example, a sample for a microscopic phytoplankton count will be fixed with lugol’s solution, a sample for zooplankton with formaline, and most samples for chemical analysis are filtered on glass fibre or membrane filters and stored in a deep freezer. Detailed explanation on these sample treatments is given in the chapters concerning the different components.