
The Atom
{ This material was found on an excellent web site under the topic: Atomic
Alchemy: Nuclear Processes. I highly recommend you visit this site if
this material interests you. http://library.thinkquest.org/17940/index.html
}
Introduction:
The atom is the
fundamental building block of all stuff, or what scientists like to call
"matter". An individual atom is very small. In fact, the smallest type
of atom, hydrogen, has a diameter of 10-8
cm. This means that if the hydrogen atom was the size of a soccer ball, then a
soccer ball would be 6450 kilometers (4008 miles) high. Every single object is
composed of atoms. Your body is made up of many, many individual atoms. There
are also many different types of atoms. In fact, there are over a 100. These
different types are called elements. Examples of some elements are hydrogen,
oxygen, iron, copper, and helium. Under normal conditions many atoms can stick
together to form larger, different stuff. Scientists call material that results
from the joining of different types of atoms "compounds". An example
of a compound is water, which is a group of two hydrogen atoms and one oxygen
atom. Notice that we said that these types of compounds can only form under what
we called "normal conditions". In the type of environment in which
nuclear fusion occurs, the joining of atoms, also known as bonding, can't
happen. We will explain why later.
|
Smaller Than the Atom:
So, are atoms made of
even smaller stuff? The answer to this question is yes. Atoms are mostly empty
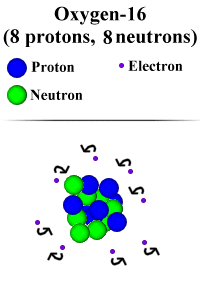
space, but in the center of the atom is a structure called a nucleus. The
nucleus is a congregation of particles. These particles are called protons and
neutrons. Neutrons are neutral, or have no electrical charge. Protons, however,
carry a positive electrical charge of 1. So, in a carbon atom, which has 6
protons in its nucleus, the overall electric charge of the nucleus would be 6.
However, a regular atom is electrically neutral. This is because swirling around
the nucleus in what is called the "electron cloud". The electrons in
the electron cloud counteract the positive charges of the protons in the atomic
nucleus with their negative electrical charges. This generates the neutral
charge of the atom. The number of electrons and number of protons correlate in a
one to one ratio. This means that there are the same number of protons and
electrons in one atom. So, if an atom has 6 protons, like carbon, it will also
have 6 electrons. The 6 electrons each have a charge of -1. This means that the
total charge of all the electrons is -6, or -1x6. The charge of carbon's nucleus
is 6 (from the protons), so when you add the two: 6 + -6, you get 0, which means
that the atom, overall, has no charge.
Note: Picture NOT to scale: An atom is more than 99% empty space,
and the protons and neutrons make up a very small amount of the volume of an
atom. Additionally, the electrons are much smaller in proportion to the nucleons
(protons and neutrons) than we have depicted. The nucleons are actually about
1800 times the size of an electron.
|
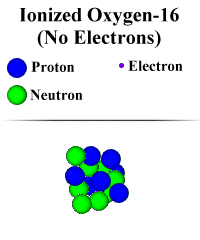
Ionization:
Before, we mentioned
that in an environment in which nuclear reactions occur, atoms don't form
groups. Scientists would say "They don't bond." So, why don't they
"bond"? When atoms bond together, they do so by sharing electrons.
Additionally, when electrons gain energy, they move out farther from the nucleus
of the atom. In some environments, there is extremely intense heat. Heat is a
form of energy, and electrons can absorb this energy. So, electrons in the atoms
become VERY excited and energetic. As a result, they move out very far away from
the nucleus. So far, in fact, that they are really not part of the atom anymore.
When this happens, we call the atom "ionized". (This is sort of like
when a young child eats a lot of sugar. He or she can get really excited and
tends to wander away from his parents!) If an atom is ionized, or has no
electrons, it can't form chemical bonds with other atoms. This is because
electrons are required for those bonds, and they aren't present because the atom
has become "ionized". One of the places this happens to an atom is in
a star.
Atomic Nomenclature
Introduction:
Throughout this web
site we will use different terminology to describe atomic processes. It is
important that you know this terminology, or "nomenclature", so that
you can understand what we are talking about! In this page we are going to
highlight some of the vocabulary you must understand.
Atomic Number:
The atomic number of
an atom is essentially the number of protons in its nucleus. For example, carbon
has 6 protons. Thus, its atomic number is 6. Earlier, we talked about isotopes.
Remember that each isotope has the same number of protons? Well, every isotope
of an element has the same atomic number.
Atomic Mass:
The atomic mass of an
element is the sum of the number of protons AND neutrons. For example, carbon
has six protons. One isotope of carbon also has 6 neutrons. This means that its
atomic mass is 12, or 6 protons + 6 neutrons. Another isotope of carbon has 8
neutrons. This means that its atomic mass would be 14, or 6 protons + 8
neutrons.
What exactly is an Isotope?
An isotope is a type
of an atom. One element can have many different isotopes. This is because an
atom is defined by its number of protons, not it's number of neutrons. Isotopes
are simply atoms of an element with different numbers of neutrons, but the same
number of protons.
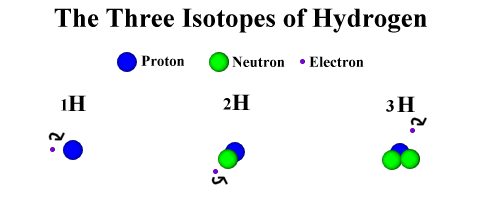
Each isotope has one proton but a
different number of neutrons.
Note: Picture Not To Scale
|
Referring to Isotopes:
Elements are often
referred to by their symbol, and not their name. For instance, we might call
Beryllium Be. (Scientists are lazy people. Why write the full name when they can
abbreviate it?) So, how do we distinguish between different isotopes? They are
the same element, after all. Well, it turns out that there is a notation that
says what type of element we're talking about AND tells the isotope. This is
done by giving the element symbol and the isotope mass in smaller text to the
left of the symbol. This is written like this: 244Pu .
Pu is the symbol for plutonium, an element used in nuclear fission. The number
244 is the atomic mass. This tells the isotope because we know that plutonium
has 94 protons. This means that we are referring to the isotope of plutonium
with 150 neutrons. 244Pu is pronounced "plutonium 244"
or "Pu 244".
Periodic Table of Elements:
The Periodic Table of
Elements is a listing of all the known elements (types of atoms) that exist.
They are listed in order of atomic number (number of protons). In our table, we
have each element's atomic number at the top of each box, its atomic symbol in
the center, and its atomic mass at the bottom. The atomic mass of each element
is not a whole number. At first, this appears to make no sense. How could the
sum of an atom's protons and neutrons be 1.01 (the atomic mass of hydrogen)? It
is impossible, after all, to have .1 neutrons or .1 protons. To understand this,
first recall that the same type of atoms can have different numbers of neutrons,
and thus different atomic masses. The reason that the atomic masses are not
whole numbers is because the atomic masses of the isotopes are averaged to get
the overall atomic mass for the element. We will explain more about this later.
For now, remember that the mass of the isotope of an element that occurs most
often in nature is the atomic mass rounded to the nearest whole number. For
example, hydrogen's (H) mass is 1.01. So, the mass of its most common isotope is
1.
Sub-Nucleonic Particles (Quarks):
| Quark
| Charge
| Up
| +2/3
| Down
| -1/3
| Strange
| -1/3
| Charm
| +2/3
| Beauty
| -1/3
| Truth
| -2/3
|
| | | | | | | |
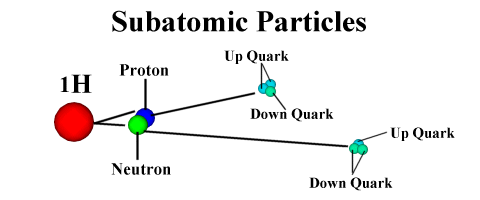
Nucleons are
neutrons or protons. Thus, sub-nucleonic particles are smaller than and compose
nucleons. These particles are called quarks. There are six types of quarks: up,
down, strange, charm, truth, and beauty. (The scientists who discovered quarks
got a little creative with their naming scheme..) The proton is composed of two
up quarks and one down quark bound together. The neutron is composed of one up
quark and two down quarks. By adding the charges of individual quarks together,
we can show the charges of the nucleons they compose. Consider the following
chart:
A proton is composed of uud (up-up-down), and a neutron is made up of udd
(up-down-down).
Proton's charge = charges of its components added = 2/3 + 2/3 + -1/3 =
1
Neutron's charge = charges of its components added = 2/3 + -1/3 + -1/3 =
0
Quarks can compose particles other than protons or neutrons. However, these
particles are very exotic and rare. They also usually have very short lives;
they often decay after less than a second. Single quarks have never been
observed, and it is believed that quarks must exist in pairs or triplets. Quarks
are thought to be fundamental particles, which means that nothing smaller
composes them. This is not proven, however. It is possible that quarks are also
composed of smaller particles.
What does this famous
and mysterious equation mean? We've all seen it, and yet a lot of us don't
understand it or its implications. Essentially, it states:
Energy = mass x c2. ; c is
the speed of light. This is an incredibly large number because light travels
extremely quickly. Think about it: When you flick a light switch, light appears
instantaneously. This is because light travels so fast from the light source to
you that it seems to take no time at all. The actual speed of light is about
300,000 kilometers a second (186,000 miles/second). If you were in an airplane
that traveled at the speed of light, you could completely circle the earth in
less than a second. That's FAST!!
Now that we know what the equation means literally, what are its
implications? Basically, it shows that energy is matter, and that they are
interchangeable. Matter can be turned into energy, and energy can be turned into
matter. This means that if a particle gains a lot of energy, it appears to have
gained mass, because energy is mass. Thus, a particle's mass depends on its
environment. If its environment is very hot (and thus has a lot of energy), it
can gain mass in the form of gained energy. Additionally, if even a small amount
of mass is converted to energy, a gargantuan amount of energy is still released.
This is because the speed of light is so large a number. For instance, we can
determine the amount of energy that composes one kilogram of mass by plugging
the numbers into the equation using the speed of light in meters/second. The
energy is measured in joules, which is the standard energy unit in the metric
system.
Energy = 1 kg x (the speed of light in meters/second)2
Energy = 1 x 300,000,0002
Energy = 90,000,000,000,000,000 joules (a metric energy unit)
This is equivalent to 25,000,000,000 kWh (kiloWatt hours) of electricity, or
25 billion kWh. This is enough electricity to power 47,000 60-watt light bulbs
for an entire year. Thus, it is quite easy to see that just one kilogram of
matter is equivalent to an enormous amount of energy. However, it turns out that
there is no practical way to convert mass completely into energy. At least, not
yet.
Anti-Matter:
You may have watched
the American TV show, "Star Trek". They talk occasionally about
anti-matter as the source of power for the ship. Well, it turns out that
anti-matter isn't just science fiction. Anti-matter is actually science fact.
But, what is it? Simply put, anti-matter is the opposite of normal matter. For
every "normal" particle of matter there is an opposite, anti-particle.
There are anti-electrons, which are just like electrons but, instead of a
negative charge, carry a positive charge. They are called positrons. There are
anti-protons and anti-neutrons, which are composed of anti-quarks that are just
like real quarks, but have opposite properties. Anti-protons are negatively
charged instead of being positively charged. Anti-neutrons are neutral, just
like normal neutrons, but have the opposite spin of a regular neutron. (Spin is
another property of particles) There are even anti-atoms. These atoms are
composed of anti-neutrons, anti-protons, and positrons (anti-electrons). So, you
might be wondering, "Have I ever seen anti-matter?". The answer is no,
and here's a tip: If you do see a lump of antimatter moving towards, you....
run. Whenever anti-matter comes in contact with matter, both masses convert
directly into energy in a violent explosion. Anti-particles don't last long on
earth because they almost instantly annihilate themselves on matter particles.
However, their presence has been recorded in specially designed machines whose
purpose is to create anti-matter, among other things. Antimatter-matter
annihilation is the only way known for atoms to completely convert to energy. It
could be a possible energy source in the future. If a 1/2 kilogram of
anti-matter could be created and stored, it could be combined with a 1/2
kilogram of regular matter to release the amount of energy equivalent to 1
kilogram, which is what we described in the above section. However, as of now,
no one knows how to make that much antimatter, and, if we could, no one knows
how to store it safely. We'll leave those problems to Star Trek and the Starship
Enterprise for now.
HoHo and Anti-HoHo Annihilation
If an Anti-Hoho and a regular Hoho
were to come in contact, they would annihilate each other. Each package
of hoho's is approximately 60 grams. Two packages (Hoho + Anti-Hoho)
would thus weigh 130 grams. This is equivalent to .130 kg.
|
Masses of the Nucleons:
We have a confession to make. We
lied to you (again). In our explanation of Atomic Nomenclature, we implied that
neutrons and protons have the same mass. This is not true. The neutron is
actually .16% bigger than the proton. This is a result of the fact that neutrons
and protons are made of different quarks. The neutron is u-d-d, while the proton
is u-u-d. The up quark has a very slight difference in mass from the down quark,
and this results in the discrepancy between the masses of the neutron and
proton. This discrepancy is really not that big, and thus is not incredibly
important when figuring the mass of an atom. It is also important to remember
that the mass of a neutron and a proton can also change with an increase or
decrease in energy of the particle. Energy is mass, after all. Additionally, the
individual nucleons in an atom give up some of their mass to hold the atom
together. This mass that is "given up" is present in the form of
energy in the nucleus. Because it binds the atom together, it is called
"binding energy". The mass each nucleon gives up is also relatively
small.
Atomic Nomenclature
A Gram of Hydrogen:
Let's say that I told
you to get a gram of hydrogen and bring it to me. How would you do that? Well,
you could go to the atom store, grab some hydrogen, and weigh it to a gram on
the nearest scale, but that would be much too easy. What if I were to tell you
that in a gram of hydrogen, there are always 6.022 x 1023
atoms? So, now all you have to do is count out that many atoms and hand em' over
to me. You better start now; it'll probably take you a while.
Moles and the Periodic Table:
In the section above
we explained how many atoms are in a gram of hydrogen. That number is a special
number called "Avogadro's Number". If you have 6.022 x 1023
atoms of any substance, we say that you have a "mole" of that
substance. If I have 6.022 x 1023 water
molecules, I have a mole of water. If I have 6.022 x 1023
cats, I have a mole of cats. (That would be a lot of cats. How would I feed
them, I wonder? I suppose I'd need a few moles of that canned cat food stuff.)
So, a mole is a unit of measurement that tells how many objects (atoms,
molecules, or... cats) you have.
Remember in the first part of Atomic Nomenclature when we explained the
periodic table and how to calculate atomic masses? Well, if you have a mole of
hydrogen atoms, you'd have a 1 gram of hydrogen, right? This is what we
explained in the first section of this page. So, let's think about this for a
second. Hydrogen has 1 nucleon, a proton. Helium, however, usually has 4
nucleons. It has 2 protons and 2 neutrons. That means that each helium atom is
about 4 times the mass of a hydrogen atom, right? It has 4 nucleons, while
hydrogen has 1. That means that if I have 2 hydrogen atoms and 2 helium atoms,
the mass of my 2 helium atoms will be 4 times that of the mass of my 2 hydrogen
atoms. So, if I have a mole of hydrogen atoms and a mole of helium atoms, the
mass of my collection of helium atoms will also be 4 times that of the mass of
my hydrogen atoms. So, if a mole of hydrogen atoms weighs 1 gram, then a mole of
helium will weigh 4 grams. Let's stop and think again. What is the atomic mass
of the main isotope of helium? It's 4. So, the mass of a mole of helium (known
as helium's molar mass) is the same as it's atomic mass. In fact, that's what
the atomic mass really does measure. The atomic masses of the atoms really
measure the mass of a mole of that atom. For example, the atomic mass of the
most common isotope of carbon is 12. Thus, if I had a mole of carbon-12, I would
have 12 grams of carbon.
Average Atomic Mass:
You've got 20 of
helium atoms sitting in a box. I tell you to find the average mass. Most of the
atoms' masses are 4(4He), but 2 are 3(3He). How
would you find the average atomic mass? Well, you would add up all the mass-4
atoms (18 of them), and then add in the mass-3 atoms (2 of them). 18x4 + 2x3 =
78. This is the sum of all their masses. To find the average mass, you need to
divide by the total number of atoms. 78/20 = 3.9. Therefore, the average mass of
the helium atoms in your box is 3.9. This is pretty simple, right? What if I
didn't tell you the number of atoms in the box, and I only told you that, say,
10% are 3He, and the rest are 4He. You could still
calculate the average in much the same way:
10% x 3 + 90%x4 = .1 x 3 + .9 x 4 = 3.9
We must then divide by 100%, which is 1, so the answer is still 3.9. Thus,
the average mass is 3.9. This is exactly how scientists calculate the average
atomic mass of an element. They don't know how many atoms of each isotope are in
the universe, but they do know the percent of each type of atom. From this, they
can calculate the average atomic mass for each element. Also, only
non-radioactive isotopes are used in calculating atomic mass.



![]()

![]()

![]()
![]()
![]()

![]()
![]()
![]()

![]()
![]()
![]()
![]()