Introduction
I. INTRODUCTION
Research into the production and investigation of clusters has grown substantially during the past few years and led, consequently, to the development of a number of new techniques. One such innovative technique based on laser vaporization within the throat of a supersonic nozzle is outlined in the following presentation. This laser vaporization scheme for producing clusters has provided an alternative to the earlier developed hot-oven based schemes allowing for an easy extension of cluster studies to even the highest boiling point materials. The capability to easily and reliably produce clusters of elements such as tungsten, chromium, and silicon has been demonstrated making this the most powerful and suitable technique for such studies.
Interest in the study of clusters initially developed due to the lack of experimental data relevant to the most fundamental physical properties (electronic structure, ionization potential, etc.) for the simplest of these species. Similar information pertaining to the larger clusters (trimer, etc.) has been even more difficult to obtain. Subsequently, interest developed because of the possibility that transition metal cluster studies could lead to greater understanding in a number of scientifically and economically important areas such as, catalysis and surface chemistry.
Historically speaking, the study of small metal clusters began in the condensed phase using the method of matrix isolation. Initial researchers included Ozin1, Moskovits2, Lindsay3, and Bondybey4. The technique involves co-condensation of metal vapors and an inert gas (Ar, Kr, etc. ) onto a cold finger at a temperature of approximately four degrees kelvin which allows for clusters to be stabilized, cooled, and then investigated spectrally. These studies had obtained an enormous amount of information but certain limitations encouraged the pursuit of alternative methods.
The extension of cluster (alkali metals) studies into the gas phase as first reported by Schumacher and co-workers5 involved the use of a hot-oven source coupled to a supersonic molecular beam. With this source in combination with mass selective photoionization detection, they obtained information about electronic structure and ionization thresholds for small sodium and potassium clusters. Though this work was a landmark in cluster studies, further advances were sought which would enable even higher boiling point metals (transition metals) to be studied.
In the effort to extend cluster studies to the transition metals, two subsequent designs were reported. The first method which was accomplished by Gole and co-workers6 involved development of a hot-oven based source capable of producing a Cw free jet expansion of pure copper vapor. The source as reported in their paper had not been sufficiently analyzed in order to determine the cluster distribution in their beam, though spectroscopic studies revealed a considerable concentration of Cu2 being produced. Riley et al.7 , next reported a source that combined a very high temperature vaporization oven with a cryogenically cooled quench cell. This scheme was successfully operated at temperatures up to 2000K, and produced Cw cluster beams of such relatively refractory metals as Al, Cr, Ni, Cu, and Ag. Unfortunately however, all of these hot oven based techniques incur the same problem with the design and construction of the equipment due to the fact that these preliminary steps must be carried out with extreme care. Another difficulty is that at these very high temperatures of 2000-6000 K contamination from surfaces and potential nozzle clogging problems may arise.
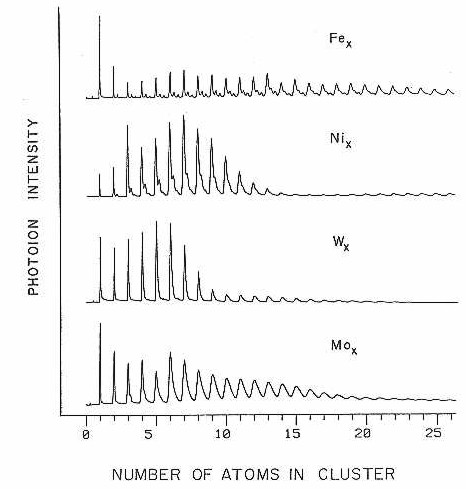
A further step in the study of clusters was made when the well-studied laser vaporization of metals8-10 (used in 1969 by Leach11,12 for the production of A12 and C2) was coupled to both supersonic expansion and quench flow configurations. The laser vaporization technique developed in our lab13-15 and also, concurrently by Bondybey16,17 , has shown that metals can be coaxed into the gas phase, clustered, and then cooled to near zero degrees kelvin. The single most valuable advantage of this technique is that only a very local heating occurs, which in contrast to the oven based schemes, considerably reduces the design and material requirements. The local heating also permits extensive cooling which preserves all of the benefits of a normal supersonic expansion. These sources have been effective in producing clusters of even the highest boiling point metals (see Figure I) with relative ease and reliability.
Figure.1
