Comments
Return to Home Page.................. Page in Progress...............
The Pennsylvania State University: Click here
Undergraduate Research __ Physical Chemistry __ Dr. R. B. Bernheim
Rice University: Click here
July 1981 to Sept 1981__Summer Intern__Dr. R. E.Smalley Lab
Sept 1981 through April 1985__Graduate Student__Robert A. Welch Predoctoral Fellow
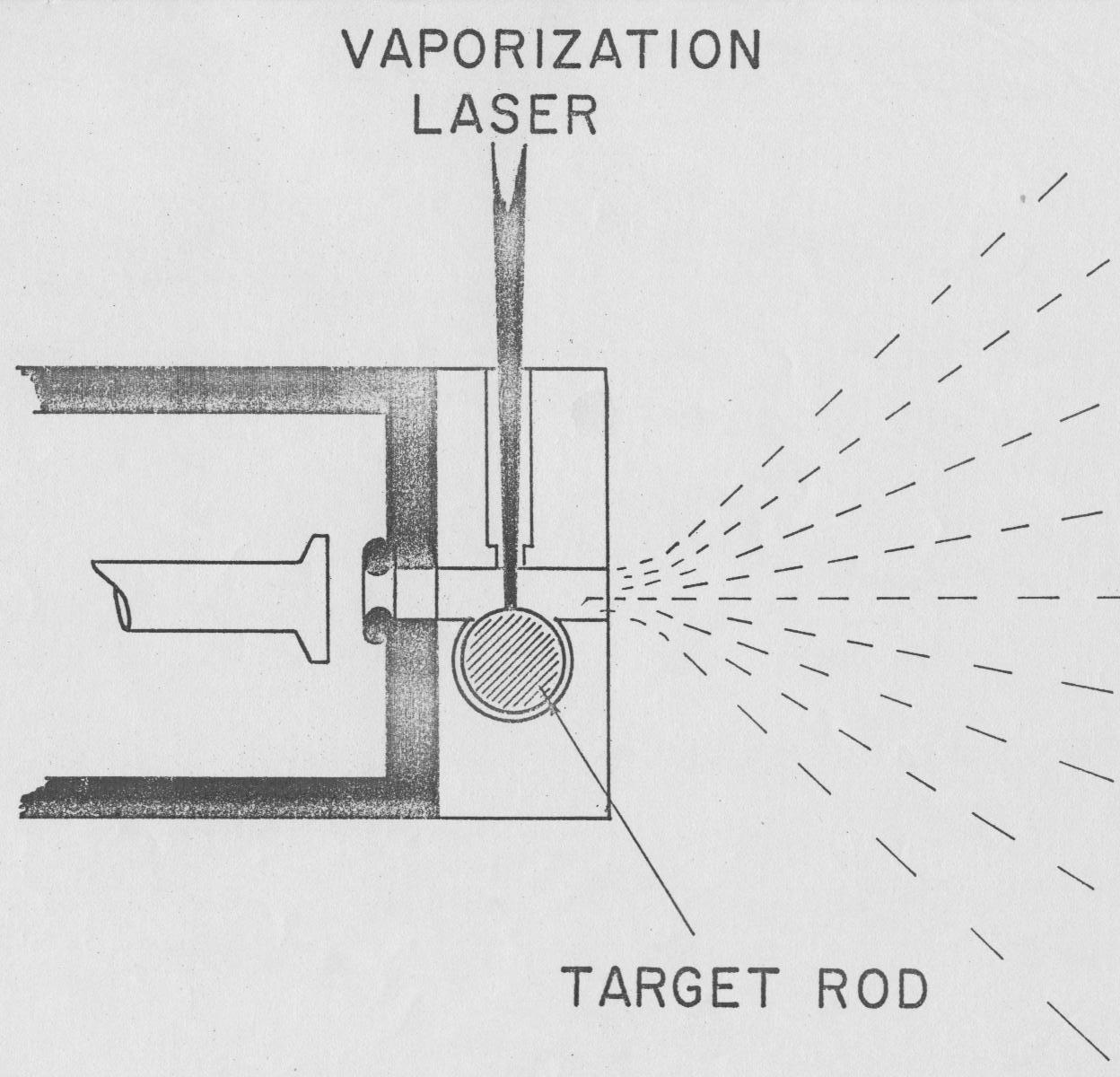
Cluster Source:
The development of the source was carried out by Dave Powers, Mike Geusic, Cristina Puiu (Talyor)and Steve Hansen on AP2. It involved the design, construction and implementation of a source based on laser vaporization that would be reliable in reproducing (pulse-to-pulse and long-term) clusters of any selected target material. J. Hopkins and P. Langridge-Smith did provide assistance in transferring the design of their version of the Grant type nozzle, constructed for a non-related experiment, to the team for further development and implementation. My most notable contribution was in realizing that metal cluster growth should be controlled by the kinetics (i.e. atom-by-atom addition and collisional stabilization of the growing species by the carrier gas). This defined the pressure and interaction time as critical parameters and helped lead to the successful construction of a reliable cluster source.
To view the publication, click here

An initial experiment by Dietz et al. [J. Chem. Phys.(1981) 74,6511] involving the accidental observation of the production of small Aln clusters ( n < 6 ) by laser vaporization of the nozzle housing , but which proved to be non-reproducible pulse-to-pulse and long-term at that time , prompted the above development. For more information concerning the initial experiment(s) see Dr. M.A. Duncan's Thesis - July 1981.
For additional details concerning the source development.
The first reasonably stable source was in place by January of 82, and although issues like coils shorting, magnetic actuators breaking etc. persisted, we were able to start the initial testing of the source using Cu clusters while over the next month or so those issues were resolved.
Copper Clusters - The initial test and characterization of the source.
Copper Dimer: Once the source had been developed to the point of being reliable and stable, copper dimer was chosen to be studied in an effort to characterize and test the source. Working with D. Powers, S. Hansen and C. Puiu, we investigated the absorption spectrum of Cu2 in the 4480-4660-A region using two-color resonant two-photon ionization. In addition to the known B <-- X band system, a new system was found in the same spectral range which was designated as the C state.
From the information obtained during this experiment the Cu cluster beam was found to have translational, rotational, and vibrational temperatures of <5, <10, and 50-100 K, respectively. Showing that the extensive cooling normally observed in a supersonic expansion was preserved and that laser vaporization was a viable technique for producing cooled cluster beams.
Note: C. Puiu's postdoctoral term ended and D. Michalopoulos having completed his Ph.D. with Dr. Elliot R. Bernstein at Colorado State University, joined the group as her replacement.
Then working with D. Powers, S. Hansen and D. Michalopoulos, we carried out an extensive survey of the ultraviolet absorption spectrum of copper dimer. Resonance two-photon ionization (R2PI) with mass selective detection allowed for the detection of an additional five new electronic band systems in the region between 2690 and 3200 A, for each of the three naturally occurring isotopic forms of Cu2.
Bracketing Ionization Potentials
Copper Clusters ranging in size from 1 to 29 atoms were produced and using a number of fixed frequency outputs of an excimer laser, the threshold behavior of the photoionization crosss section was monitored as a function of cluster size.
The two main observations are:
1) There is a pronounced even/odd alternation present up to the largest cluster studied ( 29 atoms).
2) The ionization potential has not converged to the bulk for a cluster containing 29 copper atoms.
The 7.9 eV photon energy of the F2 excimer laser was found to be above the ionization potential of all dusters, and the photoion mass spectrum thus produced showed the copper cluster concentration in the beam to follow a monotonically decreasing function of cluster size.
The 6.4 eV ArF excimer laser photon energy was found to be above the photoionization threshold of clusters with three or more atoms in the case of odd-numbered clusters, but only for clusters with eight or more atoms for even-numbered clusters. Extending out to clusters as large as 29 atoms laser photoionization at 6.4eV produced a time-of-flight mass distribution with a pronounced even/odd alternation in cluster photoion intensity. This alternation in ionization threshold behavior was attributed to an even/odd alternation in the electronic structure of the copper clusters with the highest occupied moleculer orbital (HOMO) of the even clusters being considerably more strongly bonding than it is in the clusters with an odd number of copper atoms.
The 4.98 eV photon energy of the KrF excimer laser was found to be below the ionization threshold of all clusters in the 1 to 29 atom range.
Note:
In the process of scanning the R2PI spectrum of these new electronic states, the ionization potential of the copper dimer was determined to be 7.894 eV.
Chromium Dimer
Study of the first row transition metal diatomics was undertaken in order to provide information concerning the nature of the metal-metal bond and in particular the d-orbital contribution. My most significant contribution was to propose, and working with D. Michalopoulos and S. Hansen carried out the rovibronic spectroscopic investigation of chromium dimer(4600 A band), which clearly established that the d-orbitals do play a significant role in its bonding. During this period D. Powers was busy writing his thesis, but did help when requested.
My original idea for suggesting we consider studying chromium dimer, which I presented to D.L. Michalopoulos, S.G. Hansen and M.D. Morse since Rick was on vacation hiking in Colorado with Lynn, was that since the ground state of the chromium atom is a half-filled 3d5 4s1 configuration in a high spin 7S state, the dimer could have a tightly bound ground state with a bond order possibility as high as six.
Previous to our experiment, Efremov et al.(1974) , in a flash photolysis study of chromium hexacarbonyl vapor, observed a transient absorption in the 4600 A region.. Rotational analysis of this spectra indicated a bond length of r = 1.68 A, but the analysis could not rule out the possibility of this spectrum being due to CrO2 or CrC2.
A major advantage of the resonant two-photon two-color ionization technique, we used, is that mass and spectral information are obtained simultaneously. This allowed us to determine unequivocally the carrier of the spectral feature.
Chromium Dimer - New Electronic States
During the time I was writing this thesis, I identified at least two new electronic states of chromium dimer to the red of the 4600A band, which are delineated in Appendix I.
My original assignment, as outlined in Appendix I, was for three new electronic states. I later reconsidered the original assignment and suggested a possible alternative assignment corresponding to, two new electronic states instead of three, but which could not be rigorously proven due to the lack of a high-resolution rotational spectrum for one of the lines (1-0).
High resolution rotational scans of bands for both states corresponding to the 52-52 and 50-52; isotopic species, were obtained. From the well-resolved rotational structure, it was apparent that a Q branch was not present. This type of rotational structure, consisting of a P and R branch, is what would be expected for a Σ - Σ rovibronic transition.
Both states were seen to originate from the same lower state as the 4600 A band (i.e. as shown by having a bond length, within experimental error, matching the ground state).
The lowest excited state, which I referred to as system I, with the 0-0 transition lying at 19,396.12 cm-1 + 2 was found to have a bond length of r o' = 1.635 A + .01 and a high vibrational frequency ΔG'1/2 = 574.54 cm-1 + 2. Lying to the red, 452 cm-1 of the 0-0 band is a small peak which is assigned as the 0-1. This spacing matches the ΔG"1/2=452 cm-1 as reported by Bondybey for the 4600 A band.
See Thesis page 180: Cr2 high resolution scan 0-0 transition (19,396.12 cm-1 + 2)
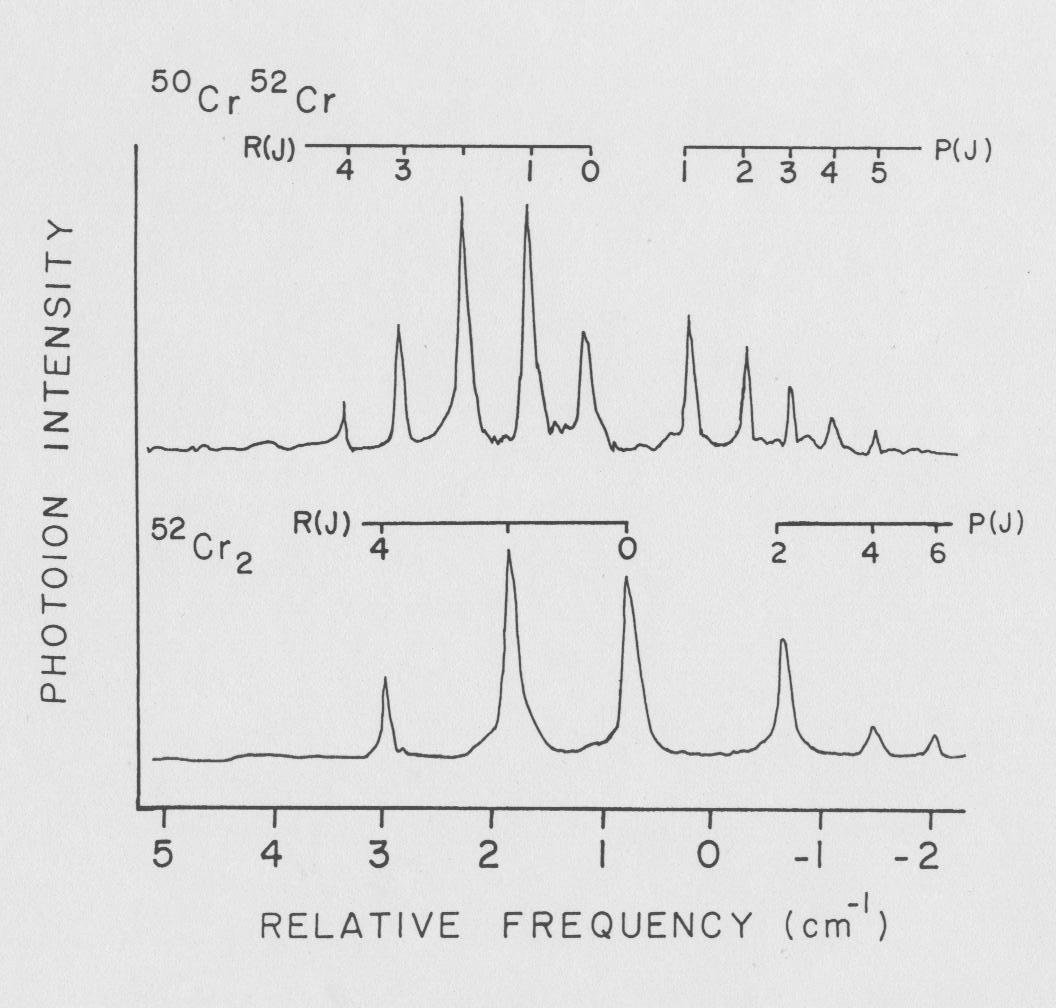
The "two state" assignment can be derived form the vibronic scan shown in my thesis (page 178) by combining the original system I and II into the new system I and re-labeling the original system III as the new system II.
Nickel Dimer
Working with M. Morse, G. Hansen, P. Langridge-Smith, Lan-Sun Zheng and D. Michalopoulos, we reported the first gas‐phase electronic spectrum of nickel dimer. Using resonant two photonionization, bands previously observed in inert matrices and attributed to Ni2 were conspicuous in their absence. Further to the red, an abrupt onset of complicated spectral structure indicates rapid predissociation above 16 680 cm− 1. We believe that this represents the true dissociation limit, and places D 0=2.068±0.01 eV. A congested pattern of spectral features from 6000 to 9000 Å confirms theoretical predictions of a large number of low‐lying electronic states in nickel dimer. Rotationally resolved bands near 8500 Å are indicative of a ΔΩ=+1 transition, with Ω″=4, Ω′=5. Rotational analysis of these bands yields a bond length of 2.200±0.007 Å for the ground state of Ni2, which must be of either 1Γ g or 3Γ u electronic symmetry species. Both the long bond length of 2.20 Å and the high value of Ω″ are in agreement with theoretical predictions, and confirm that no substantial 3d participation contributes to the chemical bonding of Ni2.
Silicon Dicarbide
In collaboration with D. Michalopoulos, we proposed and carried out the group's first non-metal cluster study involving the rovibronic spectroscopic investigation of SiC2.
It should be noted:
That P. Langridge-Smith did provide some valuable assistance in fitting the higher rotational temperature spectrum (refer to section D. Results, SiC2 page).
And that at that time Smalley was somewhat reluctant to allow the pursuit of this project due to the fact that he believed studying metal clusters was the area that would yield the most significant scientific results. Once this work was published, letters were received from at least two prominent Astrophysicists (to my knowledge) acknowledging the work and in my opinion influenced a change in thinking concerning future work.
The study of silicon dicarbide was undertaken in an effort to determine if the structure, as believed, was linear asymmetric (Si-C-C). The result of this work surprisingly did not agree with previous researchers and instead found the structure to be triangular and best described as a silicon atom bound to the side of a carbon-carbon triple bond.
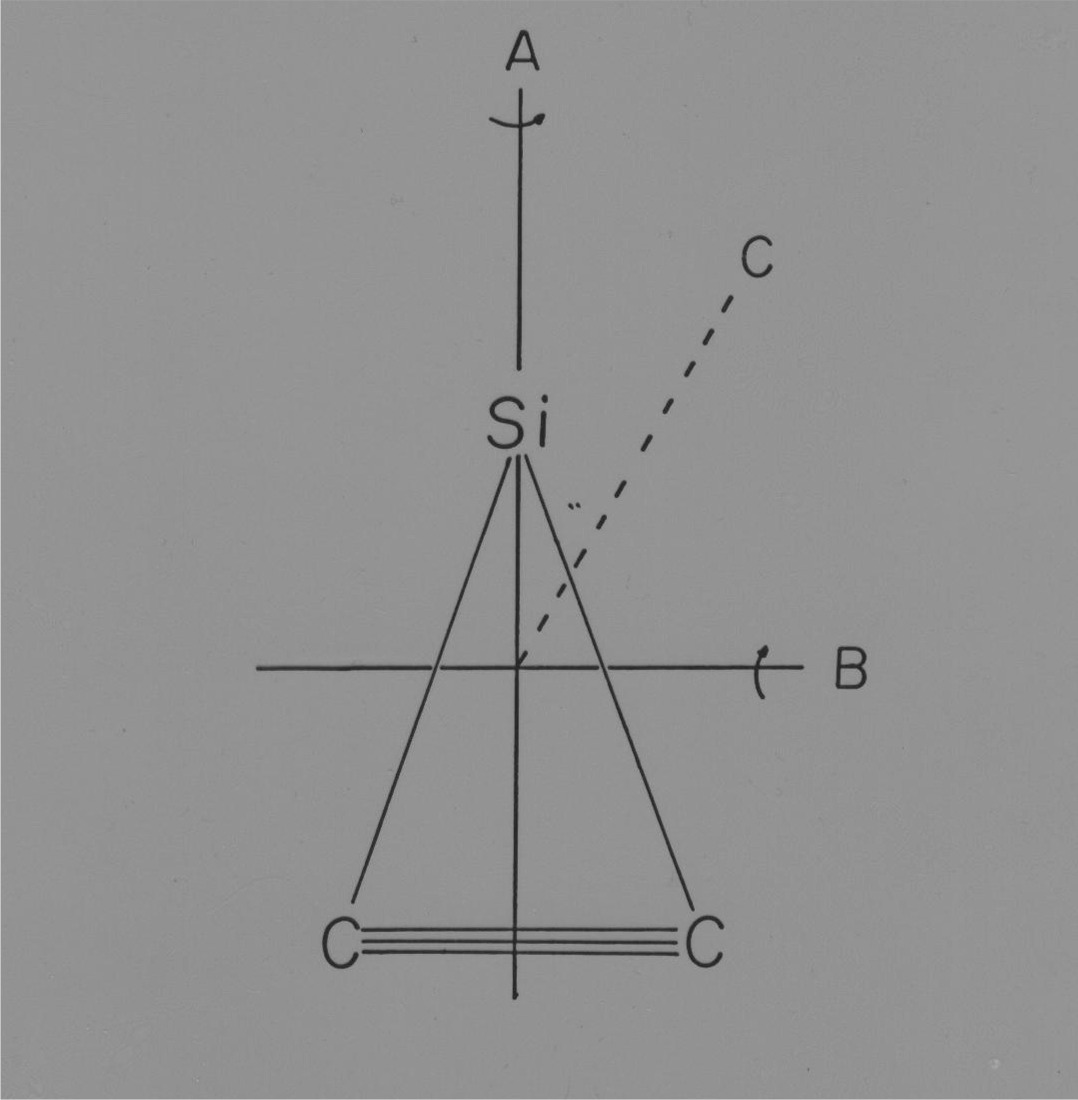
The original target of this study was silicon carbide but upon carefully searching the spectral region available in our lab we found no spectral features corresponding to this species. In retrospect, our first explanation for this result was correct. That is, SiC was not formed to any substantial amount (less than 1 % of SiC2) making the spectral signal extremely weak and therefore unobservable in our experiment.
However, we did obtain a vibronic spectra corresponding to silicon dicarbide that had been previously observed and in 1956, it was postulated, based on a partial vibrational analysis that the structure was linear asymmetric Si-C-C. In two separate investigations involving SiC2, S. Green and V. E.Bondybey, both had made observations and/or statements that questioned the then believed, Si-C-C, linear asymmetric structure. It was these comments that led us to believe that a high-resolution rotational scan of the origin could prove worthwhile.
Cluster Reaction Chemistry:
These studies entailed the design, construction and implementation of a device "the reaction tube " that could be combined with our cluster source and allow for the measurement of cluster reactivity as a function of size. Working in conjunction with M. Morse on AP2, we successfully implemented and tested "the reaction tube" allowing for these studies. Experiments were performed on clusters of various transition metals (i.e. Fe, Ni, Co, etc.) with selected reactants such as D2, N2 and CO. The results of this work showed that the reactivity of metal clusters depends on the reactant, cluster size, and metal. It also demonstrated that reactivity can change dramatically as a function of cluster size for a particular metal and reactant.
Note: A collaboration was formed in Dec. 1984 with L. Bloomfield / R. Freeman (AT&T Bell Laboratories): During Bloomfield's visit to Rice, he spent time with Rick and myself and talked about his interest to produce mass selected cluster ions of Si, etc. See below for details.
AT&T Bell Laboratories
Postdoctoral Member of Technical Staff (May 1985 through April 1987 with Dr, R. R. Freeman) Electromagnetic Phenomena Research Department
Cluster Ions:
This research involved the development of an apparatus capable of producing negative and positive cluster ions (carbon, silicon, germanium, etc.) which were then mass selected and used in photodissociation experiments. The work was an extension of a collaboration formed in Dec. 1984 with L. Bloomfield, following which I became his replacement. These experiments resulted in new information regarding photofragmentation patterns as a function of cluster size and were used to infer relative stability and possible structures. My most notable contribution to this collaboration was to provide L. Bloomfield with a first hand look and knowledge of the cluster source / experimental set-up and to suggest we investigate carbon in order to address some of the questions that arose from earlier works (i.e. Pitzer et. al. and Rohlfing et. al.)
The initial experiments of this collaboration involved producing neutral clusters that were then photoionized, mass selected and photodissociated using 532 nm light. This work focused on the photodissociation of mass selected silicon and germanium cluster cations ( n=2 to 12 ) and one particular carbon cluster cation C60+. The results for silicon (Sin+ ) showed the dominant photodissociation channel to be the loss of a neutral atom up to and including n=8, although for n=8 the loss of Si2 was seen as a strong secondary channel. Above n=8 the loss of a neutral molecular fragment was observed to be the dominate channel. For C60+ the loss of a neutral C2 was observed.
In an effort to refine our experiments (i.e. to eliminate possible misleading intensity information due to the lack of photoionization cross section data as a function of cluster size and/or fragmentation, and remove the possible introduction of additional internal energy due to fragmentation upon ionization) the apparatus was reconfigured allowing for ion clusters, cation and anion, to be produced directly in the source, cooled upon expansion, mass selected and then photodissociated using 248 nm light. These experiments involved the photodissociation of carbon cluster cations and identified a unique dissociation channel producing Cn-3+ and the neutral fragment C3 for clusters containing between 6-20 atoms. In addition, from the data obtained concerning photofragmentation cross sections at 248 nm, we suggested that the abrupt change between n=9 and n=10 may well be a manifestation of the predicted change (by Pitzer et. al.) from linear chains to monocyclic rings. M. Jarrold became involved in the later stages of this project , while awaiting the delivery of his apparatus to Bell Labs , helping with acquiring data, data analysis and making a major contribution to the writing of the second paper.
Studies concerning the photofragmentation of Sbn+ and Bin+ cluster cations ( n < 8 ) were also performed at Bell Labs, in collaboration with then assistant Prof. M. Duncan of the University of Georgia. The results showed the fragmentation products and branching ratios are consistent with the production of stable neutral and cation molecular fragments, suggesting a statistical dissociation mechanism.
Above Threshold Ionization (ATI):
An apparatus was assembled and experiments conducted on the above threshold ionization of xenon using subpicosecond laser pulses. The purpose of this study was to investigate the transition from the long to extremely short pulse regime using pulse widths ranging from 15 to .4 psec of 616 nm light. Our results showed how the short pulse regime develops and a new phenomenon below 1 psec was observed. I was responsible for the vacuum system including the construction and implementation of the time of flight electron spectrometer and data acquisition system. H. Milchberg was responsible for the subpicosecond laser system and together we carried out this series of experiments. My most significant contribution was to suggest that the ATI spectra indicated, due to the break-up of the low energy ATI peaks into a series of narrow peaks, some type of resonance might be taking place. This observation was then modeled by P. Bucksbuam and R. Freeman confirming that the excited states were playing a critical role. S. Darack did provide valuable assistance.
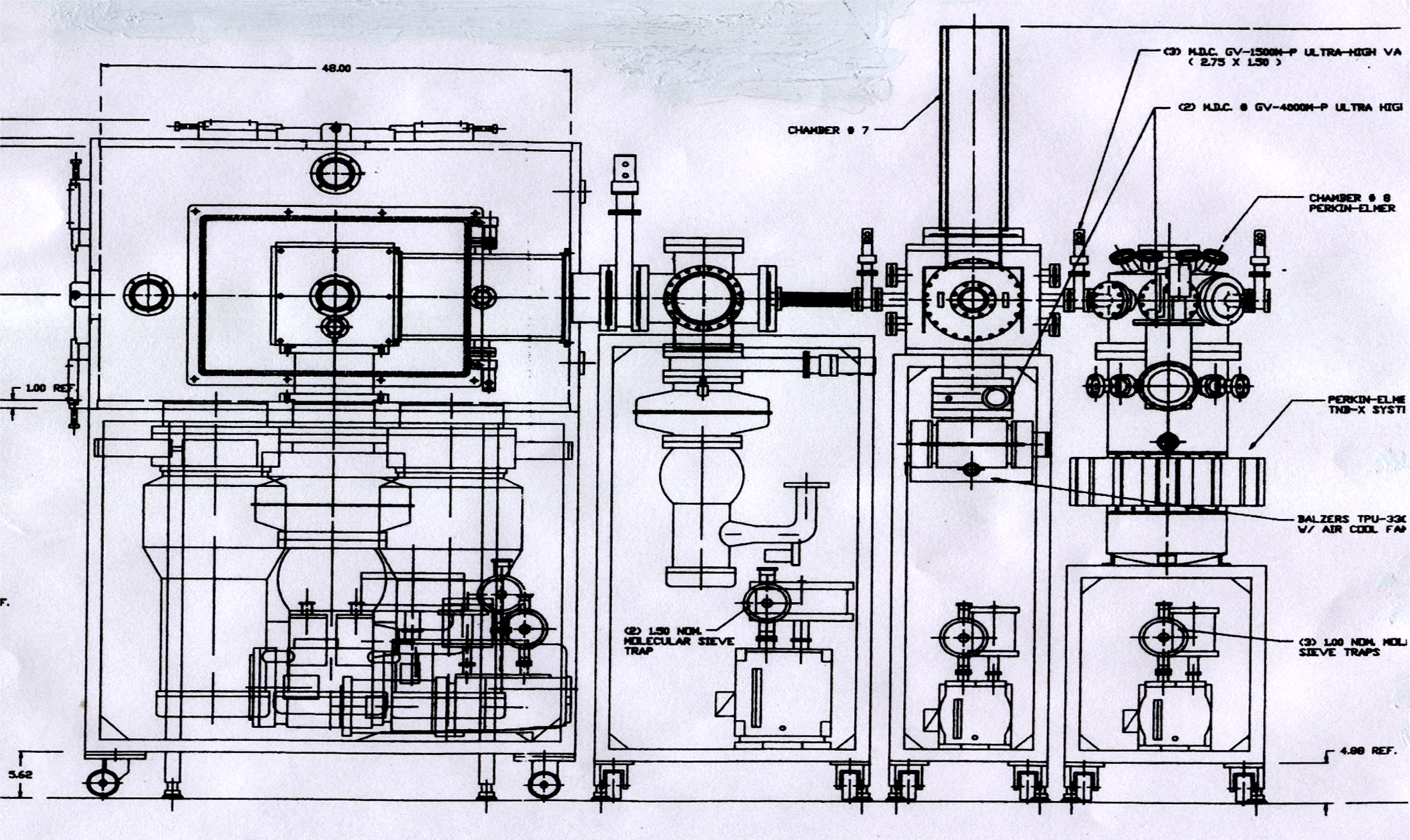
New Cluster Apparatus:
I designed and oversaw the construction (by an outside contractor) of a more sophisticated cluster apparatus that would allow for increased beam intensity as well as expanded capabilities and flexibility, i.e. cluster deposition under UHV conditions. The delivery of the apparatus to Bell Labs came just as my two-year term ended. S. Darack did provide valuable assistance.
.
Pacific Northwest National Laboratory: - Contractor Battelle
Battelle is a Not-for-Profit meaning the company wants to maximize it's profit. Not to be confused with a Non-profit.
For this particular section, I am going to first present a series of true short stories concerning PNL (now PNNL) and the contractor Battelle
To be continued....