![]() +
+ ![]()
![]()
![]() n +
?
n +
?
| (a)
|
(b)
|
(c)
|
(d)
|
(e)
|
1) What is the missing isotope in the following nuclear reaction?
![]() +
+ ![]()
![]()
![]() n +
?
n +
?
| (a)
|
(b)
|
(c)
|
(d)
|
(e)
|
2) The half-life of an unknown isotope is 15.0 h. How many hours will it take for an initially observed activity to decrease to 25% of its initial value?
| (a) 6.2 | (b) 30. | (c) 11.3 | (d) 7.5 | (e) 5.0 |
3) ![]() undergoes a beta decay followed by an alpha decay. What is the final product?
undergoes a beta decay followed by an alpha decay. What is the final product?
(a)
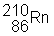 |
(b)
|
(c)
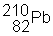 |
(d)
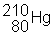 |
(e)
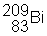 |
4) A proton captures a neutron forming a deuterium nucleus. Using the following masses, determine the mass of the deuterium nucleus.
mass
of a proton = 1.6726 x 10-27 kg, mass
of a neutron = 1.6606 x 10-27 kg |
| (a) 3.3332 x 10-27 kg | (b) more than 3.3332 x 10-27 kg | (c) less than 3.3332 x 10-27 kg |
5) What is the missing product in the following nuclear reaction?
![]() +
+ ![]()
![]()
![]() +
?
+
?
| (a) α | (b) β+ | (c) γ | (d)
|
(e)
|
6) If the half-life of I-131 is eight days, what was the mass of the original sample if 5 g of I-131 remain after 72 days?
| (a) 2560 g | (b) 2880 g | (c) 360 g | (d) 50 g | (e) 40 g |
7) A rock contains 1.37 g of Sr-90. If the half-life of Sr-90 is 29 years, what fraction of the atoms would remain 175 years later?
| (a) 0.062 | (b) 0.015 | (c) 0.50 | (d) 0.125 | (e) 0.166 |
8) Using the data below, calculate the energy released in J·mol-1 when one mole of Po-214 decays into Pb-210 and He-4.
masses: Pb-210 = 209.98284 g, Po-214 = 213.99519 g, He-4 = 4.00260 g |
| (a) 8.78 x 1011 | (b) 8.78 x 1014 | (c) 7.20 x 1014 | (d) 1.46 x 109 | (e) 9.75 x 1013 |
9) If the number of protons in a nuclide increases and the mass number remains constant, which of the following occurred?
(a) alpha decay
(b) gamma emission
(c) electron capture
(d) beta decay
10) If the mass of Be-9 is 9.012182 g/mol, what is its binding energy in J/mol?
mass
of a proton = 1.007825 g/mol, mass
of a neutron = 1.008665 g/mol |
| (a) 5.54 x 1012 | (b) 5.62 x 1012 | (c) 8.12 x 1011 | (d) 2.93 x 1011 | (e) 3.08 x 1012 |