Kinetics Of A Reaction - Calculating Activation Energy
The rate constant of a reaction can be expressed as
k = Ae-Ea/RT
which is called the Arrhenius equation. Taking the natural log of both sides of the Arrhenius equation gives
ln k = -Ea/R(1/T) + ln A
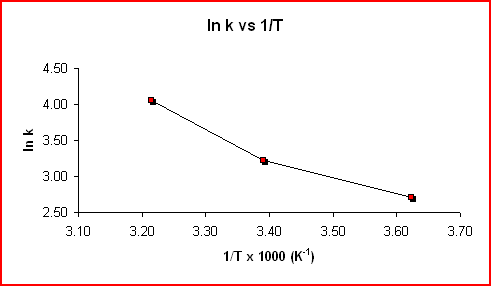
The equation above is of the form y = mx + b, where y = ln k, m = -Ea/RT, x = 1/T, and b = ln A. For a reaction whose rate constant obeys the Arrhenius equation, a plot of ln k vs 1/T gives a straight line and it's slope can be used to determine Ea.
Sample data shown in the following table was used to produce the two graphs.
Temp |
Temp-1 |
Average Time |
Rate Of Reaction |
Rate Constant (k) |
ln k |
|---|---|---|---|---|---|
(K) |
(K-1) |
(s) |
(M/s) |
(M-3s-1) |
() |
277 |
3.61 x 10-3 |
289 |
4.8 x 10-8 |
15 |
2.7 |
295 |
3.39 x 10-3 |
173 |
8.1 x 10-8 |
25 |
3.2 |
311 |
3.22 x 10-3 |
74 |
1.9 x 10-7 |
58 |
4.1 |
 |
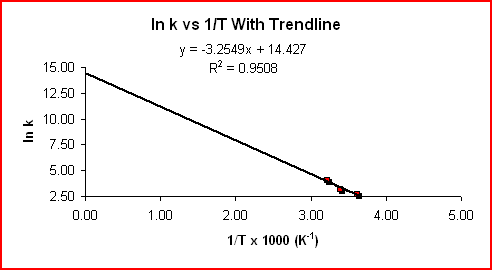 |
Using the slope from the graph displaying the trendline:
Slope = -3.2549 K x 1000 = -3300 K
Slope = -Ea/R
Ea = -Slope x R = -(-3300
Kx 8.314 J mol-1K-1)Ea = 27000 J mol-1 = 27 kJ mol-1
©2004, Flinn Scientific, Inc. All Rights Reserved. Reproduced for one-time use with permission from Flinn Scientific, Inc., Batavia, IL, USA. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc.; and
That the Licensee agrees to indemnify and hold Flinn Scientific, Inc. harmless from any and all liability, loss, damages, costs of expense which Flinn Scientific, Inc. may hereafter incur, suffer or be required to pay by reason of such publication by the Licensee; and
To compensate Flinn Scientific, Inc. $0.00 for the permission to reproduce this material.