Alimentary tract and pancreas
Alimentarni trakt i pankreas
ARCH GASTROENTEROHEPATOL 2001; 20 ( No 3 – 4
):
Review
The entroinsular axis
Enteroinsularna osovina
( accepted July 10th, 2001 )
1Tatjana Radosavljevic, 2Vera Todorovic, 3Anna JudithNikolic, 1Branka Sikic
1Institute of Pathophysiology, School of Medicine, University of Belgrade,
2Institute for Medical Research, Department of Experimental Pathology and Cytology, University of Belgrade,
3INEP – Institute for the Application of
Nuclear Energy, Zemun-Belgrade, 4Institute of Pathology,
School of Medicine, University ogf Belgrade, Belgrade, Serbia,
Yugoslavia.
Address correspondence to:
Docent Dr Tatjana Radosavljevi}
Institute of Pathophysiology,
School of Medicine, University of Belgrade,
Dr Subotica 1/II St.
YU-11000 Belgrade, Serrbia
Yugoslavia
FAX + 381 11 685 340
E-mail: [email protected]
……………………….. ………………………………
Enteroinsular axis Gastroenteroloska sekcija SLD-
O1711, 2001.
ABSTRACT
The entero-insular is a network of neural and endocrine communications between the alimentary tract and the pancreatic islets, which promotes insulin release in response to feeding. This review article describe of the major hormones of the enteroinsular axis, and mechanism of actions of this hormones.
Key words: enteroinsular axis, gut hormones, pancreatic islet of Langerhans
Sa`etak
Enteroinsularna osovina je mre`a endokrine i neuralne komunikacije izme|u gastrointestinalnog trakta i ostrvaca pankreasa koja omogu}ava osloba|anje insulina u zavisnosti od unosa hrane. U ovom revijskom ~lanku opisani su glavni hormoni enteroinsularne osovine i mehanisam dejstva.
Kljucne reci: enteroinsularna osovina, crevni hormoni, Langerhansova ostrvca.
The importance of gut-endocrine pancreas interaction on glucose
metabolism was first reported by Claude Bernard about a century ago
and outlined by Zunz and La Barre who used the term incretin as a
hypoglycemic factor in the extract of duodenum (1). With increasing
knowledge of the influence of the gut on the function of the
pancreatic islet, Unger and Eisentraut 1969 unify these relations
in the single concept: "enteroinsular axis" (2). The
entero-insular axis is a network of neural and endocrine
communications between the alimentary tract and the pancreatic
islets, which promotes insulin release in response to feeding. This
axis comprises variety of different interactions of the small
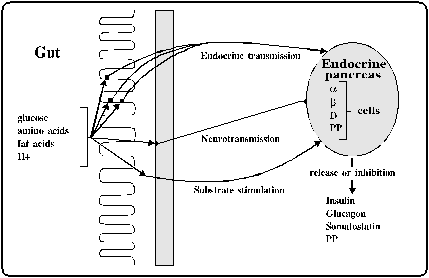
intestine with the function of the islets of Langerhans. Figure
1. The different interactions can be of endocrine or neural
origin, or caused directly by nutrients absorbed from the gut.
Incretin concept encompasses GI hormonal interaction with
glucose-induced insulin release from the pancreas. The components
of the enteroinsular system, which are basic for the incretin
effect, are the endocrine transmission in combination with
substrate stimulation of the pancreatic islet by glucose (3, 4).
Glucose-induced insulin release from pancreatic b-cells is
modulated by a number of hormones and neurotransmitters, which may
act as inhibitors and potentiators on b-cells function. In Table
1 is demonstrated localization, response to glucose and effect
on insulin release of the gut hormones. Although the majority of
hormones belonging to the secretin/glucagon family potentate
glucose-stimulated insulin release, only two of all GI hormones are
potent incretin: gastric inhibitory polypeptide (GIP) and
glucagon-like peptide-1 (GLP-1) (5,6). Both GIP and GLP-1 stimulate
the secretion of insulin in the pancreatic b-cells and are
therefore, likely to play important roles in the regulation of
postprandial glucose homeostasis in man. GLP-1 represents one of
the most potent endogenous peptides, which stimulates
glucose-dependent insulin secretion (6, 7, and
8).
Glucagon-like peptide-1 (GLP-1)
If one consider GLP-1 as an incretin, this would imply that physiological increments of GLP-1 plasma concentrations are accompanied by evidence of stimulated insulin secretion during the postprandial phase of physiological hyperglycemia (9,10). Insulin release after plasma glucose concentration rise is greater when GLP-1 secretion is stimulated by the ingestion of various nutrients such as carbohydrates, glucose, fat and mixed meal and by the administration of various gut peptides and neurotransmitters including bombesin, enkephalin, galanin and GIP (11,12, 13, 14, 15).
If hypersecretion of insulin stimulated by incretin hormones is continuos, this may cause hypoglycemia. W.Creutzfeldt proposed a negative feed back control as a fail-safe system, of incretin hormones secretion by insulin (3). GLP-1 could be regulated by the negative feed back inhibition by hyperglycemia and hyperinsulinemia. Therefore, GLP-1 secretion could be regulated by blood glucose level and insulin (16). The interdependence between glucose and incretin actions involves a cross talk between glycolysis and cAMP signaling pathways of the activated GLP-1 receptor (17). Glucose competence concept has been used to describe the mutual interdependence between glucose metabolism and GLP-1 actions on b-cells (glucose is required for GLP-1 action and GLP-1 is required to render b-cells competent to respond to glucose) (9). Mechanism of action of GLP-1 is presented in Figure 2.
Glucagon-like peptid-1 (GLP-1) is gut hormone from the incretin family, which stimulates insulin secretion, and plays an important role as primary hormonal regulator of the entero-insular axis (3, 18,19,20). GLP-1 stimulates insulin secretion during hyperglycemia and suppresses glucagon secretion (20, 21). However, it is uncertain whether GLP-1 inhibits glucagon secretion by direct actions on a-cells or indirectly by its paracrine-inhibitory effects of insulin and somatostatin on a-cells (22). This perfectly fits with concept of intraislet hormonal interaction (7). GLP-1 stimulates transcription of proinsulin gene and biosynthesis of insulin. Recent evidence indicates that GLP-1 may stimulate proliferation and neogenesis of b-cells (9). Moreover, GLP-1 decreases gastric emptying rate and acid secretion, and has pro-satiety effect (23, 24). On fat, muscle and liver, GLP-1 induce lipogenesis and glycogenesis ("insulinomimetic" actions of GLP-1). GLP-1 is an excellent candidate for the treatment of patients with type 2 diabetes mellitus (9, 10, 19, 25, 26).
GLP-1 is released into the portal circulation in response to a meal. This hormone exhibits insulinotropic action when combined with tGLP-1 pancreatic islet receptors. The hepatic vagal nerve branches, which possess specific neural tGLP-1 receptors, may fix tGLP-1 from the portal blood through a mechanism mediated by specific receptor for this hormone. When this occur, hepatic vagal afferent neural activity is facilitated/augmented what leads to further facilitation/augmentation of efferent vagal neural activity in its pancreatic vagal branches, revealing a novel vagal hepatopancreatic reflex pathway. The neural reception of tGLP-1 involves a receptor mechanism distinct from that in the well-known humoral insulinotropic action. tGLP-1 plays a role as neuroincretin in the enteroinsular axis, which is in concordance with concert of its role as humoral incretin (27, 28, 29).
Leptin, the obesity hormone produced by cells from the adipose tissue, has opposite actions to GLP-1 on pancreatic b-cells. Leptin suppresses insulin secretion and gene expression. The feedback loop between leptin and insulin constitutes an adipoinsular axis that operates physiologically in parallel with the enteroinsular axis, which involves GLP-1 and insulin (9). Disruption of either axis lead to glucose intolerance. Leptin inhibits glucose- and GLP-1-stimulated insulin secretion by activation of phosphodiesterase 3B on b-cells (30).
Glucose-dependent insulinotropic polypeptide
(GIP)
GIP is a peptide produced by K-cells of the mammalian proximal small intestine, which stimulates insulin release in the presence of hyperglycemia. Using GIP (7-30)-N which is specific receptor antagonist of naturally occurring GIP, inhibition of insulin release occur thus indicating that GIP may have a dominant role in mediating postprandial insulin secretion (5). GIP together with other gut hormones or meal stimulated neurotransmission, might contribute to enchanted insulin secretion. Under conditions of pathologic hyperglycemia, GIP may act a "fail-safe" mechanism (31). GIP has been found to be a very weak stimulator of somatostatin secretion. D-cells seem to be less sensitive to GIP than the b-cells, and the D-cells are much less sensitive to GIP, than to GLP-1. This phenomenon might explain the contrasting effects of GIP and GLP-1 on glucagon release (7).
Cholecystokinin (CCK)
The gut hormone, CCK is released from the intestinal I-cells after meal and in pancreatic islets. In pancreatic islets, CCK activates specific CCK-receptors and stimulates insulin secretion (32). CCK stimulate insulin secretion through an effect which involves mediation by phospholipase C (PLC) and protein kinase C (PKC), and partially mediated by a pathway involving phospholipase A2 (PLA2) with subsequent formation of arachidonic acid (AA) (33).
Insulinotropic action of the gastrointestinal neuropeptides: vasoactive intestinal polypeptide (VIP), and gastric releasing peptide (GRP)
VIP is localized in ganglia and nerve fibers of many organs, including gut and pancreas, but not in epithelial cells, and therefore acts only as neurotransmitter. VIP causes a prompt increase of insulin secretion, which is followed by a prolonged continuation of increased release (34).
GRP is confined to intrapancreatic nerves of the islets and the ganglia. This peptide acts as an intrapancreatic neurotransmitter, stimulating insulin release both directly through an action on the b-cells and indirectly through activation postganglionic cholinergic nerves by an action at the ganglionic level (35).
Somatostatin
Somatostatin (SS) is the brain-gut hormones, distributed in CNS and peripheral nervous systems as well as in the pancreas and gastrointestinal tract (36, 37). SS-28, secreted into the circulation from enterocytes after food, and SS-14, released from gastric and pancreatic D cells, and enteric neurons inhibit the release of a variety of peptides and neurotransmitters (38). SS-28 is potent inhibitor of insulin release, with a high affinity for b-cells (39). SS-28 participates in the enteroinsular axis as a decretin to regulate postprandial insulin secretion that controls enchanced insulin release in response to absorbed nutrients and secreted incretins during to early phases of alimentation. This leads to proper regulation of waning of insulin to avoid unwanted hypoglycemia (38).
Splanchnic organs are known to be the major source of
circulating SS. It suggests that peptide hormones secreted from the
splanchnic organs acts on its own specific receptor on the afferent
vagal nerve in the hepatoportal area. This neural monitoring system
which important parts are SS and GLP-1, convert humoral information
to neural information through the unique receptor mechanism in the
hepatoportal area (40).
Secretin
The secretin-producing cells are localized in the proximal part of the small intestine. Intraluminal secretion concentrations go up in the interdigestive period following a mixed meal. Secretin stimulates glucose-induced insulin release. Moreover, this hormone exhibited a glucose-dependent stimulation of glucagon and somatostatin secretion (4). Secretin-induced glucagon release could implicate glucagon as an intermediate paracrine or endocrine hormone in the potentiation of insulin release.
Glucose-induced insulin release from the pancreatic b-cell is controlled by three regulatory pathways involving changes in cytosolic Ca2+ concentration, intracellular concentration of cAMP, diacylglicerol and inositol phosphates depending on the activation of protein kinase A, B and C. Secretin stimulated glucose-dependent insulin release by interacting with the intracellular cAMP-dependent pathway (4).

|
Figure 2. Mechanism of action of GLP-1
|
Figure 1. Entero-insular axis
(Creutzfeldt and Ebert, 1986; Kofod, 1992)
Table 1. Familyes of gastrointestinal hormones
|
Family |
Localization |
insulin release |
released by glucose |
|||
|
Secretin / glucagon |
||||||
|
Secretin |
intestine |
yes |
yes |
|||
|
Glucagon |
Pankreas |
yes |
no |
|||
|
GLP-1 (7-36) amid |
intestine |
yes |
yes |
|||
|
GLP-2 |
intestine |
no |
yes |
|||
|
GIP |
intestine |
yes |
yes |
|||
|
VIP |
intestine |
yes |
||||
|
PHI |
intestine |
yes |
||||
|
Cholecystokinin / gastrin |
||||||
|
Cholecystokinin |
intestine |
yes |
no |
|||
|
Gastrin |
intestine |
yes |
Very litle |
|||
|
PP group |
||||||
|
PP |
pancreas |
|||||
|
PYY |
intestine |
no |
||||
|
NPY |
brain |
no |
||||
|
Tachikinins |
||||||
|
Substance P |
intestine |
inhibits |
yes |
|||
|
Opioids |
intestine |
yes |
||||
|
Bombesin – GRP |
intestine |
yes |
||||
|
Somatostatin |
intestine |
inhibits |
yes |
|||
|
|
pancreas |
REFERENCES - LITERARURA:
Zunz E, La Barre J: Contributions a l’etude des variations physiologiques de la secretion interne du pancreas. relations entre les secretions externe et interne du pancreas. Arch Int Physiol Biochim 1929; 31: 20-44.
2. Unger RH, Eisentraut AM: Entero-insular axis. Arch Intern Med 1969; 123: 261-6.
3. Creutzfeldt W, Ebert R: The enteroinsular axis. in: the exocrine pancreas - biology, pathobiology and diseases, Govl W et al. (eds).. Raven Press, New York, 1986 333-46.
4. Kofod H: Secretin and the endocrine pancreas. Acta Endocrinol 1992; 126: 12-41.
5. Tseng CC, Kieffer TJ, Jarboe LA, Usdin TB, Wolfe MM: Postprandial stimulation of insulin release by glucose-dependent insulinotropic polypeptide. J Clin Invest 1996; 98: 2440-5.
6. Holst JJ: Enteroglucagon. Annu Rev Physiol 1997; 59: 257-71.
7. Fehmann HC, Goke R, Goke B: Cell and molecular biology of the incretin hormones glucagon-like peptide-1 and glucose-dependent insulin releasing polypeptide. Endocrine Rev 1995; 16: 390-410.
8. Kolligs F, Fehmann HC, Goke R, Goke B: Reduction of the incretin effect in rats by the GLP-1 receptor antagonist exendin. Diabetes 1995; 44: 16-19.
9. Kieffer TJ, Habener JF. The glucagon-like peptides. Endocrine Reviews 1999; 20: 876-913.
10. Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res 2001; 56: 377-99.
11. Fieseler P, Bridenbaugh S, Nustede R, Martell J, Orskov C, Holst JJ, et al. Physiological augmentation of amino acid-induced insulin secretion by GIP and GLP-1 but not by CCK-8. Am J Physiol 1995; 268: 949-55.
12. Shimizu I, Hirota M, Ohboshi C, Mizuno A, Shima K: Effect of the enteroinsular axis on both the a- and b-cell response to arginine after oral glucose man. Diabetologia 1987; 30: 846-50.
13. Hermann-Rinke C, Horsch D, McGregor GP, Goke B: Galanin is a potent inhibitor of glucagon-like peptide-1 secretion from rat ileum. Peptides 1996; 17: 571-6.
14. Hermann-Rinke C, Voge A, Hess M, Goke B: Regulation of glucagon-like peptide - 1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol 1995; 147: 25-31.
15. Ritzel U, Fromme A, Ottleben M, Leonhardt U, Ramadori G: Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol 1997; 34: 18-21.
16. Sugiyama K, Manaka H, Yamatani K, Tominaga M, Sasaki H: Regulation of glucagon-like peptide-1 from the isolated perfused canine ileum by circulating glucose and insulin. Biomed Res 1996; 17: 373-7.
17. Holst JJ: Glucagon, glucagon-like peptide-1 and their receptors: an introduction. Acta Physiol Scand 1996; 157: 309-15.
18. Wang Z, Wang RM, Owjii AA, Smith DM, Ghatei MA, Bloom SR: Glucagon-like peptide -1 is a physiological incretin in rat. J Clin Invest 1995; 1: 417-21.
19. Rachman J, Gribble FM, Barow BA, Levy JC, Buchanan KD, Turner RC: Normalization of insulin responses to glucose by overnight infusion of glucagon-like peptide-1 (7-36) amide in patients with niddm. Diabetes 1996; 45: 1524-30.
20. Orskov C: Glucagon -like peptide-1, a new hormone of the entero-insular axis. Diabetologia 1992; 35: 701-11.
21. Orskov C, Wettergren A, Holst JJ: Secretion of the incretin hormones glucagon -like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol 1996; 31: 665-70.
22. Ding WG, Renstrom E, Rorsman P, Buschard K, Gromada J: Glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide stimulate ca2+ induced secretion in rat alpha-cells by a protein kinase a-mediated mechanism. Diabetes 1997; 46: 792-800.
23. Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, et al. Glucagon-like peptide-1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 1997; 273: E981-E8.
24. Donahey JC, Van Dijk G, Woods SC, Seeley RJ: Intraventricular GLP-1 reduces short- but not long-term food intake or body weight in lean and obese rats. Brain Res 1998; 779: 75-83.
25. Gefel D, Barg Y, Zimlichman R: Glucagon-like peptide-1 structure, function and potential use for niddm. Isr J Med Sci 1997; 33: 690-5.
26. Perfetti R, Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol 2000; 143: 717-25.
27. Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A: The hepatic vagal nerve is receptive to incretin hormone glucagon - like peptide - 1, but not to glucose - dependent insulinotropic polypeptide, in the portal vein. J Autonom Nerv Syst 1996; 61: 149-54.
28. Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A: Vagal hepatopancreatic reflex effects evoked by intraportal appearance of GLP-1. Am. J. Physiol 1996; 271: 808-13.
29. Nishizawa M, Nakabayashi H, Kawai K. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. Auton Nerv Syst 2000; 80: 14-21.
30. Zhao AZ, Bornfeldt KE, Beavo JA: Leptin inhibits insulin secretion by activation of phosphodiesterase 3b. J Clin Invest 1998; 102: 869-73.
31. Sarson DL, Wood SM, Kansal PC, Bloom SR: Glucose-dependent insulinotropic polypeptide augmentation of insulin. physiology or pharmacology? Diabetes 1984; 33: 389-93.
32. Liddle RA: Cholecystokinin cells. Annu Rev Physiol 1997; 59: 221-42.
33. Simonsson E, Karlsson S, Ahren B: Involment of phospholipase A2 and arachidonic acid in cholecystokinin-8-induced insulin secretion in rat islets. Regul Pept 1996; 65: 101-7.
34. Sharp GW: Mechanism of inhibition of insulin release. Am J Physiol 1996; 271: 1781-99.
35. Karlsson S, Sundler F, Ahren B: Insulin secretion by gastrin-releasing peptide in mice: ganglionic versus direct islet effect. Am J Physiol 1998; 274: 124-9.
36. Di Scala-Guenot D, Strosser MT, Mialhe P: Characterization of somatostatin in peripheral and portal plasma in the duck: in vivo metabolism of somatostatin-28 and -14. J Endocrinol 1984; 100: 329-35.
37. Chiba T, Yamada T: Gut somatostatin. in: gut peptides - biochemistry and physiology, Walsh JH, Dockray GJ (eds). Raven Presss, New York, 1994: 123-45.
38. Ensinck JW, Vogel R, Laschansky E, Koerker D, Prigeon R, Kahn SE, et al. Endogenous somatostatin-28 modulates postprandial insulin secretion: immunoneutralization studies in baboons. J Clin Invest 1997; 100: 2295-302.
39. Draznin B: Somatostatin receptors in endocrine cells. The Receptors 1985; 2: 401-22.
40. Nakabayashi H: Neural monitoring system for circulating somatostatin in the hepatoportal area. Nutrition 1997; 13: 225-9.