 Introduction
to Turbines
Introduction
to Turbines Introduction
to Turbines
Introduction
to Turbines
Steam Turbines Gas Turbines Thermodynamics
![]()
Turbines
Turbine, rotary engine that converts the energy of a moving stream of water, steam, or gas into mechanical energy. The basic element in a turbine is a wheel or rotor with paddles, propellers, blades, or buckets arranged on its circumference in such a fashion that the moving fluid exerts a tangential force that turns the wheel and imparts energy to it. This mechanical energy is then transferred through a drive shaft to operate a machine, compressor, electric generator, or propeller. Turbines are classified as hydraulic, or water, turbines, steam turbines, or gas turbines. Today turbine-powered generators produce most of the world's electrical energy. Windmills that generate electricity are known as wind turbines
The success of the water turbine inevitably led to consideration of the turbine principle for extracting power from steam. Where the Watt-type reciprocating steam engine utilized the pressure of steam, the turbine could achieve higher efficiency by utilizing the kinetic energy of steam flow. The turbine can be made smaller, lighter, and cheaper than a reciprocating steam engine of comparable power. It can be made in far larger sizes than the conventional steam engine. Mechanically, it has the advantage of producing rotating motion directly without the necessity of using a crankshaft or other means of transforming reciprocal to rotary motion. As a result, the steam turbine has supplanted the reciprocating engine as a prime mover in large electricity-generating plants and is also used as a means of jet propulsion.
Steam turbines are used in the generation of nuclear power and in nuclear ship propulsion. They operate with fuel-fired boilers for power generation. In cogeneration applications requiring both process heat (heat used in an industrial process) and electricity, steam is raised at high pressure in the boiler and extracted from the turbine at the pressure and temperature required by the process. Steam turbines may be used in combined cycles with a steam generator which recovers heat that would otherwise be lost. Industrial units are used to drive machines, pumps, compressors, and electrical generators. Ratings range from a few horsepower to more than 1300 Mw.
The steam turbine was not invented by any one individual but was the result of work by a number of inventors in the latter part of the 19th century. Notable contributors to the development of the turbine were the British inventor Charles Algernon Parsons and the Swedish inventor Carl Gustaf Patrik de Laval. Parsons was responsible for the so-called principle of staging, whereby steam was permitted to expand in a number of stages, performing useful work at each stage. De Laval was the first to design suitable jets and blades for the efficient use of the expanding steam.
The action of the steam turbine is based on the thermodynamic principle that when a vapor is allowed to expand, its temperature drops, and its internal energy is thereby decreased. This reduction in internal energy is transformed into mechanical energy in the form of an acceleration of the particles of the vapor (see Thermodynamics). This transformation makes a large amount of work energy directly available. In the case of expanding steam, a reduction of 100 Btu in internal energy through expansion can result in increasing the speed of the steam particles to a rate of almost 2900 km/h (almost 1800 mph). At such speeds the energy available is great, even though the particles are extremely light.
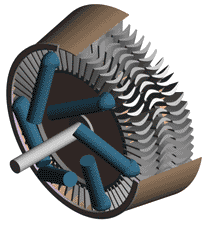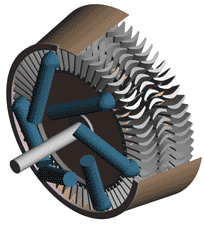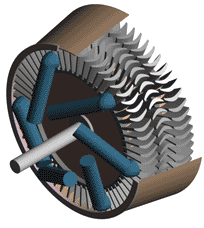
Although they are built according to two different principles, the essential parts of all steam turbines are similar. They consist of nozzles or jets through which steam flows and expands, dropping in temperature, and gaining kinetic energy, and blades against which the swiftly moving steam exerts pressure. The arrangement of jets and blades, whether fixed or stationary, depends upon the type of turbine. In addition to these two basic components, turbines are equipped with wheels or drums upon which the blades are mounted, a shaft for these wheels or drums, an outer casing that confines the steam to the area of the turbine proper, and various pieces of auxiliary equipment, including lubrication devices and governors.
The simplest form of steam turbine is the so-called impulse turbine, in which the turbine jets are fixed in place on the inside of the turbine casing, and the blades are set on the rims of revolving wheels mounted on a central shaft. Steam passing through a fixed nozzle passes over the curved blades; these absorb some of the kinetic energy of the expanded steam, turning the wheel and shaft on which they are mounted. The turbine is designed so that steam entering at one end of the turbine expands through a succession of nozzles until it has lost most of its internal energy.
In the reaction turbine, mechanical energy is obtained to some degree by the impact of steam upon the blades, but primarily it is obtained by the acceleration of the steam as it expands. A turbine of this type consists of a set of fixed and a set of movable blades. The blades are arranged so that each pair acts as a nozzle through which the steam expands as its passes. The blades of a reaction turbine are usually mounted on a drum and not on a wheel. This drum acts as the shaft of the turbine.
In order to use the energy available in steam efficiently in a turbine of either type, it is necessary to employ a number of stages, in each of which a small amount of thermal energy is converted to kinetic energy. If the entire conversion of energy took place instead in a single expansion stage, the rotative speed of the turbine wheel would be excessive. In general, reaction turbines require more stages than impulse turbines. It can be shown that for the same diameter and energy range, a reaction turbine requires twice the number of stages for peak stage efficiency. Large turbines that are nominally of the impulse variety employ some reaction at the root of the steam path to assure efficient flow through the buckets. Many turbines that are nominally reactive have an impulse control stage first, which allows for a saving in the total number of stages.
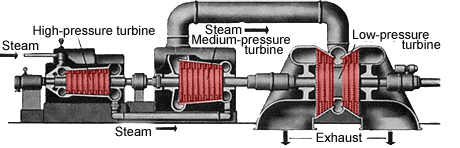
Because of the increase in volume as the steam expands through the various stages of a turbine, the size of the openings through which the steam passes must increase from stage to stage. In the practical engineering design of turbines, this increase is accomplished by lengthening the blades from stage to stage and by increasing the diameter of the drum or wheel upon which the blades are mounted and by adding two or more turbine sections in parallel. As a result, a small industrial turbine may be more or less conical in shape, with its smallest diameter at the high-pressure, or inlet, end, and its largest at the low-pressure, or exhaust, end. A large unit for a nuclear power station may have four rotors consisting of one double-flow high-pressure section followed by three double-flow low-pressure sections.
Impulse turbines usually employ pressure or Rateau staging, named after the French engineer Auguste Rateau, in which the pressure ratio across each stage is nearly uniform. Impulse turbines built in the past have made use of velocity-compounded, or Curtis, staging named after its American inventor, Charles Gordon Curtis, which has two sets of moving buckets with an intermediate set of fixed blades following the nozzles. The staging of a reaction turbine may be called Parsons' staging, after its British inventor, Charles Parsons.
Steam turbines are comparatively simple machines, having only one major moving part, the rotor; however, auxiliary equipment is necessary for their operation. Journal bearings support the shaft. A thrust bearing positions the shaft axially. An oil system provides lubrication to the bearings. Seals minimize steam leakage within the steam path. A sealing system prevents steam leaking from the machine and air leaking from the outside into the machine. The speed of rotation is controlled by valves at the inlet(s) of the machine. In addition, reaction turbines develop considerable axial thrust owing to the pressure drop across the moving blades. This is usually compensated for by the use of a dummy piston, which creates a thrust in the opposite direction to that of the steam path.
The expansion efficiency of a modern multistage steam turbine is inherently high because of the state of development of the steam-path components and the ability to recover losses of one stage in those downstream through reheating. The efficiency with which a section of a turbine converts the theoretically available thermodynamic energy to mechanical work is commonly in excess of 90 percent. The thermodynamic efficiency of a steam-power installation is much less, owing to the energy lost in the exhaust steam from the turbine.
Gas Turbine, also combustion turbine, engine that employs gas flow as the working medium by which heat energy is transformed into mechanical energy. Gas is produced in the engine by the combustion of certain fuels. Stationary nozzles discharge jets of this gas against the blades of a turbine wheel. The impulse force of the jets causes the shaft to turn. A simple-cycle gas turbine includes a compressor that pumps compressed air into a combustion chamber. Fuel in gaseous or liquid-spray form is also injected into this chamber, and combustion takes place there. The combustion products pass from the chamber through the nozzles to the turbine wheel. The spinning wheel drives the compressor and the external load, such as an electrical generator.
In a turbine or compressor, a row of fixed blades and a corresponding row of moving blades attached to a rotor is called a stage. Large machines employ multistage axial-flow compressors and turbines. In multishaft arrangements, the initial turbine stage (or stages) powers the compressor on one shaft while the later turbine stage (or stages) powers the external load on a separate shaft.
The efficiency of the gas-turbine cycle is limited by the need for continuous operation at high temperatures in the combustion chamber and early turbine stages. A small, simple-cycle gas turbine may have a relatively low
thermodynamic efficiency, comparable to a conventional gasoline engine. Advances in heat-resistant materials, protective coatings, and cooling arrangements have made possible large units with simple-cycle efficiencies of 34 percent or higher.
The efficiency of gas-turbine cycles can be enhanced by the use of auxiliary equipment such as intercoolers, regenerators, and reheaters. These devices are expensive, however, and economic considerations usually preclude their use.
In a combined-cycle power plant, the considerable heat remaining in the gas turbine exhaust is directed to a boiler called a heat-recovery steam generator. The heat so recovered is used to
raise steam for an associated steam turbine. The combined output is approximately 50 percent greater than that of the gas turbine alone. Combined cycles with thermal efficiency of 52 percent and higher are being put into service. Gas turbines have been applied to the propulsion of ships and railroad locomotives. A modified form of gas turbine, the turbojet, is used for airplane propulsion. Heavy-duty gas turbines in both simple and combined cycles have become important for large-scale generation of electricity. Unit ratings in excess of 200 megawatts (MW) are available. The combined-cycle output can exceed 300 MW.
The usual fuels used in gas turbines are natural gas and liquids such as kerosene and diesel oil. Coal can be used after conversion to gas in a separate gasifier.
Thermodynamics, field of physics that describes and correlates the physical properties of macroscopic systems of matter and energy. The principles of thermodynamics are of fundamental importance to all branches of science and engineering.
A central concept of thermodynamics is that of the macroscopic system, defined as a geometrically isolable piece of matter in coexistence with an infinite, unperturbable environment. The state of a macroscopic system in equilibrium can be described in terms of such measurable properties as temperature, pressure, and volume, which are known as thermodynamic variables. Many other variables (such as density, specific heat, compressibility, and the coefficient of thermal expansion) can be identified and correlated, to produce a more complete description of an object and its relationship to its environment.
When a macroscopic system moves from one state of equilibrium to another, a thermodynamic process is said to take place. Some processes are reversible and others are irreversible. The laws of thermodynamics, discovered in the 19th century through painstaking experimentation, govern the nature of all thermodynamic processes and place limits on them.
II. Zeroth Law of Thermodynamics
The vocabulary of empirical sciences is often borrowed from daily language. Thus, although the term temperature appeals to common sense, its meaning suffers from the imprecision of nonmathematical language. A precise, though empirical, definition of temperature is provided by the so-called zeroth law of thermodynamics as explained below.
When two systems are in equilibrium, they share a certain property. This property can be measured and a definite numerical value ascribed to it. A consequence of this fact is the zeroth law of thermodynamics, which states that when each of two systems is in equilibrium with a third, the first two systems must be in equilibrium with each other. This shared property of equilibrium is the temperature.
If any such system is placed in contact with an infinite environment that exists at some certain temperature, the system will eventually come into equilibrium with the environment—that is, reach the same temperature. (The so-called infinite environment is a mathematical abstraction called a thermal reservoir; in reality the environment need only be large relative to the system being studied.)
Temperatures are measured with devices called thermometers (see Thermometer). A thermometer contains a substance with conveniently identifiable and reproducible states, such as the normal boiling and freezing points of pure water. If a graduated scale is marked between two such states, the temperature of any system can be determined by having that system brought into thermal contact with the thermometer, provided that the system is large relative to the thermometer.
III. First Law of Thermodynamics
The first law of thermodynamics gives a precise definition of heat, another commonly used concept.
When an object is brought into contact with a relatively colder object, a process takes place that brings about an equalization of temperatures of the two objects. To explain this phenomenon, 18th-century scientists hypothesized that a substance more abundant at higher temperature flowed toward the region at a lower temperature. This hypothetical substance, called "caloric," was thought to be a fluid capable of moving through material media. The first law of thermodynamics instead identifies caloric, or heat, as a form of energy. It can be converted into mechanical work, and it can be stored, but is not a material substance. Heat, measured originally in terms of a unit called the calorie, and work and energy, measured in ergs, were shown by experiment to be totally equivalent. One calorie is equivalent to 4.186 × 107 ergs, or 4.186 joules.
The first law, then, is a law of energy conservation. It states that, because energy cannot be created or destroyed—setting aside the later ramifications of the equivalence of mass and energy (see Nuclear Energy)—the amount of heat transferred into a system plus the amount of work done on the system must result in a corresponding increase of internal energy in the system. Heat and work are mechanisms by which systems exchange energy with one another.
In any machine some amount of energy is converted into work; therefore, no machine can exist in which no energy is converted into work. Such a hypothetical machine (in which no energy is required for performing work) is termed a "perpetual-motion machine of the first kind." Since the input energy must now take heat into account (and in a broader sense chemical, electrical, nuclear, and other forms of energy as well), the law of energy conservation rules out the possibility of such a machine ever being invented. The first law is sometimes given in a contorted form as a statement that precludes the existence of perpetual-motion machines of the first kind.
IV. Second Law of Thermodynamics
The second law of thermodynamics gives a precise definition of a property called entropy. Entropy can be thought of as a measure of how close a system is to equilibrium; it can also be thought of as a measure of the disorder in the system. The law states that the entropy—that is, the disorder—of an isolated system can never decrease. Thus, when an isolated system achieves a configuration of maximum entropy, it can no longer undergo change: It has reached equilibrium. Nature, then, seems to "prefer" disorder or chaos. It can be shown that the second law stipulates that, in the absence of work, heat cannot be transferred from a region at a lower temperature to one at a higher temperature.
The second law poses an additional condition on thermodynamic processes. It is not enough to conserve energy and thus obey the first law. A machine that would deliver work while violating the second law is called a "perpetual-motion machine of the second kind," since, for example, energy could then be continually drawn from a cold environment to do work in a hot environment at no cost. The second law of thermodynamics is sometimes given as a statement that precludes perpetual-motion machines of the second kind.
V. Thermodynamic Cycles
All important thermodynamic relations used in engineering are derived from the first and second laws of thermodynamics. One useful way of discussing thermodynamic processes is in terms of cycles—processes that return a system to its original state after a number of stages, thus restoring the original values for all the relevant thermodynamic variables. In a complete cycle the internal energy of a system depends solely on these variables and cannot change. Thus, the total net heat transferred to the system must equal the total net work delivered from the system.
An ideal cycle would be performed by a perfectly efficient heat engine—that is, all the heat would be converted to mechanical work. The 19th-century French scientist Nicolas Léonard Sadi Carnot, who conceived a thermodynamic cycle that is the basic cycle of all heat engines, showed that such an ideal engine cannot exist. Any heat engine must expend some fraction of its heat input as exhaust. The second law of thermodynamics places an upper limit on the efficiency of engines; that upper limit is less than 100 percent. The limiting case is now known as a Carnot cycle.
VI. Third Law of Thermodynamics
The second law suggests the existence of an absolute temperature scale that includes an absolute zero of temperature. The third law of thermodynamics states that absolute zero cannot be attained by any procedure in a finite number of steps. Absolute zero can be approached arbitrarily closely, but it can never be reached.
VII. Microscopic Basis of Thermodynamics
The recognition that all matter is made up of molecules provided a microscopic foundation for thermodynamics. A thermodynamic system consisting of a pure substance can be described as a collection of like molecules, each with its individual motion describable in terms of such mechanical variables as velocity and momentum. At least in principle, it should therefore be possible to derive the collective properties of the system by solving equations of motion for the molecules. In this sense, thermodynamics could be regarded as a mere application of the laws of mechanics to the microscopic system.
Objects of ordinary size—that is, ordinary on the human scale—contain immense numbers (on the order of 1024) of molecules. Assuming the molecules to be spherical, each would need three variables to describe its position and three more to describe its velocity. Describing a macroscopic system in this way would be a task that even the largest modern computer could not manage. A complete solution of these equations, furthermore, would tell us where each molecule is and what it is doing at every moment. Such a vast quantity of information would be too detailed to be useful and too transient to be important.
Statistical methods were devised therefore to obtain averages of the mechanical variables of the molecules in a system and to provide the gross features of the system. These gross features turn out to be, precisely, the macroscopic thermodynamic variables. The statistical treatment of molecular mechanics is called statistical mechanics, and it anchors thermodynamics to mechanics.
Viewed from the statistical perspective, temperature represents a measure of the average kinetic energy of the molecules of a system. Increases in temperature reflect increases in the vigor of molecular motion. When two systems are in contact, energy is transferred between molecules as a result of collisions. The transfer will continue until uniformity is achieved, in a statistical sense, which corresponds to thermal equilibrium. The kinetic energy of the molecules also corresponds to heat and—together with the potential energy arising from interaction between molecules—makes up the internal energy of a system.
The conservation of energy, a well-known law of mechanics, translates readily to the first law of thermodynamics, and the concept of entropy translates into the extent of disorder on the molecular scale. By assuming that all combinations of molecular motion are equally likely, thermodynamics shows that the more disordered the state of an isolated system, the more combinations can be found that could give rise to that state, and hence the more frequently it will occur. The probability of the more disordered state occurring overwhelms the probability of the occurrence of all other states. This probability provides a statistical basis for definitions of both equilibrium state and entropy.
Finally, temperature can be reduced by taking energy out of a system, that is, by reducing the vigor of molecular motion. Absolute zero corresponds to the state of a system in which all its constituents are at rest. This is, however, a notion from classical physics. In terms of quantum mechanics, residual molecular motion will exist even at absolute zero. An analysis of the statistical basis of the third law goes beyond the scope of the present discussion.